Zinc Acetate Dihydrate: A Down-to-Earth Look
Historical Development
Chemists have turned to zinc compounds for centuries. Zinc acetate’s use dates back to simpler pharmacy days, when it turned up as a mild astringent or a wound-healing agent. Over time, laboratories refined its processing, developing the crystalline dihydrate form. Before chemical companies nailed down cleaner, more reliable methods, people relied on trickier, less pure sources. Now labs and factories count on its distinct structure, shaped by years of slow but steady progress and hands-on tinkering from both researchers and industry workers. This trail from small-scale pharmacy bottles to large drums for global industries didn’t just happen by chance. People gained ground step by step, testing each improvement.
Product Overview
Zinc acetate dihydrate gets a spot in everything from lozenges on pharmacy shelves to reagents in classrooms. Much of the world’s supply comes from converting zinc oxide or metal using acetic acid under controlled conditions. Tablets, feed supplements, chemical baths, textiles—credible sources still list new uses as technology changes. One reason for that is its reliable supply chain and the fact that it stores well in a dry environment. Standard containers protect it from lumping and keep its crystalline form looking clean and regular, a sign that producers know what end users need.
Physical & Chemical Properties
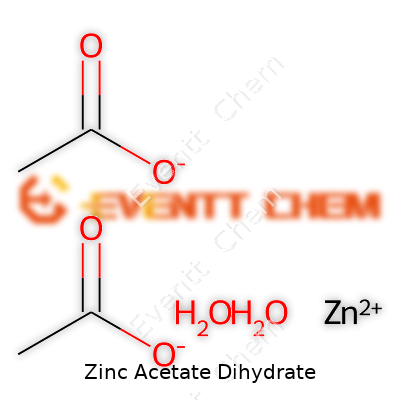
The best way to recognize zinc acetate dihydrate is through its colorless crystals, with a sweet but slightly vinegar-like smell. At room temperature, it melts into a liquid at 237 °C and dissolves readily in water. Just by working with the stuff on a bench, you notice it sticks and clumps up if the humidity runs high. This characteristic comes in handy where solubility makes a difference, such as in textile mordants or lab reagents. On the chemical side, it brings both zinc and acetate ions to the table and delivers predictable results in applications where purity matters. The formula Zn(CH3COO)2·2H2O says as much about its practicality as any description can. With zinc ions tightly bound and water of crystallization in the structure, it stays stable for long storage spells.
Technical Specifications & Labeling
Labs and end users expect labels to include purity, water content, heavy metal limits, and storage advice. Manufacturers refer to pharmacopeia monographs and technical data sheets, providing batch numbers and production dates. Most regulations demand minimum zinc content above 29% on a dry basis, and a moisture content consistent with the dihydrate form. Producers stamp containers with hazard pictograms, usually flagging irritation risk without making it seem more dangerous than it is. These essentials help buyers compare grades quickly, so they don’t waste time tracking down product certificates. In my experience, missing details on a label cause headaches for buyers, so completeness stands out.
Preparation Method
Start with zinc oxide, add glacial acetic acid, and then stir the mix gently at room temperature. The slow fizz signals zinc acetate formation. With water present, the solution crystallizes as the dihydrate when cooled. Manufacturers filter and dry the crystals, usually in well-ventilated setups. Scaling this up in industry calls for controlled temperature and careful choice of raw materials to dodge impurities. Trouble often arises if acid isn’t pure or if zinc oxide carries heavy metals. Most producers run purity checks at the end, since even small impurities downgrade product quality and narrow its application range.
Chemical Reactions & Modifications
Zinc acetate dihydrate acts as a mild Lewis acid and swaps ligands with other carboxylates and amines. Organic chem labs rely on it for introducing zinc ions or prepping metallic zinc catalysts. It reacts with sulfur sources to yield zinc sulfide in pigment production. Adding bases causes precipitation of zinc hydroxide, which transforms to zinc oxide with heating. Some researchers modify its structure with chelating agents, tuning it for specialty polymerization or nanoparticle synthesis. The range of chemical reactions makes it far more than a one-trick pony.
Synonyms & Product Names
Over the years, different sources have called it Zinc diacetate dihydrate, Acetic acid zinc salt dihydrate, or just plain “zinc acetate crystals.” Even trade names crop up for pharmaceutical and animal nutrition grades. In the chemical trade, warehouse staff and truckers wind up giving it nicknames too. Reading a supply invoice or a lab protocol, you recognize these synonyms because mistakes happen if you can’t match the name with what’s inside the drum.
Safety & Operational Standards
Though not among the most hazardous chemicals, zinc acetate dihydrate demands respect. Getting it on skin can cause mild irritation, and inhaling dust means you should get to fresh air. I’ve found that chemical-proof gloves and a dust mask work just fine when weighing or mixing at the bench. Facilities keep Material Safety Data Sheets handy, not because of danger but out of habit. Spill cleanup uses water and good ventilation. Standards for pharma and food grades require more documentation and stricter adherence to Good Manufacturing Practice. Kids’ science labs should keep it locked away and labeled, but professional users rarely face surprises if they’re careful and don’t get sloppy.
Application Area
Pharmaceuticals make wide use of zinc acetate dihydrate, with lozenges and supplements proving especially popular for addressing zinc deficiency and supporting immune health. Textile manufacturers turn to it as a mordant, anchoring dyes to natural fibers like cotton. Chemical industries rely on it in catalysts, synthetic intermediates, and plating baths. Animal feed producers blend it as a nutritional supplement for livestock. Even in the classroom, teachers use it for demonstrating double-replacement reactions. The list keeps growing because every year, technical bulletins and patents describe new uses. It remains versatile for both large-volume production and niche tasks, linking traditional utility to modern technology.
Research & Development
University teams, startup labs, and major industrial players all look at zinc acetate dihydrate for exploration. Nanotechnology researchers use it to build zinc oxide nanoparticles under mild conditions. Polymer chemists see it as a cross-linking agent for certain plastics, while energy scientists test it for enhancing battery materials. I’ve worked with students who use it for growing crystals, analyzing metal chelation, or prepping catalysts. The stream of journal articles never really stops, as researchers frequently circle back to simple compounds with hidden power. Connecting the dots between old reagents and new breakthroughs drives real learning.
Toxicity Research
Toxicologists agree the risks from zinc acetate dihydrate grow with big doses. Swallowing or breathing in too much can upset gastrointestinal function, cause vomiting, or trigger zinc fever symptoms. Exposure guidelines set occupational limits to keep workers below risky levels. Medical journals record that oral supplementation within proper limits stays safe for adults. The World Health Organization and similar bodies publicly list benchmarks for maximum daily intake. Safety research keeps moving forward, with studies tracking absorption, metabolism, and environmental persistence. Experience on the floor shows that sticking to safety protocols and using up-to-date equipment keeps accidental exposure low. For hobbyists or small labs, safe use starts with simple training.
Future Prospects
Looking ahead, new materials design and environmental remediation could push demand for zinc acetate dihydrate. Pressure keeps growing for greener chemical processes and advanced battery components, both fields where this compound holds promise. Pharmaceutical producers will likely stick with it for its known benefits and manageable side effects. More countries tightening their purity standards means quality suppliers with a track record will stand out. As nanotechnology grows, the need for well-characterized zinc sources will push researchers back to basics, where zinc acetate dihydrate stands ready. Industry and academia both depend on trustworthy raw materials, so its role as a building block may only grow in the decades ahead.
In the Pharmacy: Zinc’s Role in Wellness
People ask about the real-life uses of zinc acetate dihydrate, and the conversation usually starts in the pharmacy aisle. Doctors often recommend zinc acetate lozenges to help shorten the length of colds. Research from the Cleveland Clinic and work by Dr. Harri Hemilä highlight how zinc, in certain forms, can cut the duration of colds by a day or more. Our immune system loves zinc, and using the acetate form seems to make sure the body absorbs it well, without the stomach aches that come with some other zinc salts. You’ll spot it in supplements and throat lozenges because it’s safe, and the body doesn’t struggle to use it.
Wound Healing and Skin Health
After a batch of stubborn acne or a surgical cut, doctors sometimes recommend creams containing zinc acetate dihydrate. Hospitals, clinics, and even home first aid kits stock ointments and creams with this mineral because it helps wounds heal faster. The body taps into zinc’s powers for cell repair and inflammation control. Studies, including those in the Journal of the American Academy of Dermatology, support using zinc-based products for fighting bacteria and soothing irritated skin. People with chronic skin conditions or ulcers see real improvements with creams using this safe compound.
Protecting Our Food: Zinc in Animal Nutrition
Farmers add zinc acetate dihydrate to animal feed. Chickens, pigs, and cattle need balanced minerals, just like humans. Feed manufacturers use zinc acetate because it dissolves in water and mixes easily, speeding up the absorption process so animals get the right dose. The World Health Organization points out that zinc deficiency stunts animal growth and weakens immune response, so mixing this blend into feed keeps herds healthy.
Zinc Acetate Dihydrate in the Lab
School labs, universities, and research centers stash this compound in the chemicals cabinet for several reasons. Chemists use it when they grow crystals or test for the presence of sulfide ions in solutions. Researchers working on nano-materials and battery technologies include zinc acetate dihydrate in their experiments. Its predictable behavior and purity level mean fewer unexpected results—which saves time, and in science, time and accuracy both matter.
Environmental Cleanup Work
Zinc acetate dihydrate wears another hat in the fight against pollution. Environmental engineers turn to it when removing impurities from industrial wastewater. Combining it with other chemicals helps capture and trap toxic substances so wastewater can be treated before it flows back into rivers or streams. The United States Environmental Protection Agency lists zinc compounds as useful in taking phosphates and heavy metals out of water supplies.
Human Safety and Awareness
Some people might feel nervous seeing chemical names on supplement bottles or feed sacks. Zinc acetate dihydrate earns its spot because it works reliably and its safety record stretches back decades. Of course, overdosing can cause problems—nausea, or lower copper absorption, for example. So, sticking to recommended doses is common sense. Health agencies, including the US Food and Drug Administration, set those guidelines to keep consumers and animals safe.
Paving the Way for Smarter Use
Looking forward, public education can cut down on confusion about minerals like zinc acetate dihydrate. Labels that explain the “why” instead of just listing ingredients help people make smart choices at the checkout or when treating minor illness at home. In clinics, staff training includes the reasons behind ingredient choices in medications and ointments, which builds trust. In classrooms, chemistry teachers give students hands-on experiences so the next generation knows the practical value of compounds like this one.
The Power of Simplicity: Zn(CH3COO)2·2H2O
Zinc acetate dihydrate, with its formula Zn(CH3COO)2·2H2O, often shows up in labs, classrooms, and even medicine cabinets. People see this name and can easily miss the role it plays in daily life and science. For anyone who ever saw white tablets in a sore throat remedy, there’s a good chance zinc acetate is behind their metallic taste and subtle healing effect. The formula looks neat, but it packs a bigger meaning than just letters strung together.
Everyday Encounters with Chemistry
Back in high school, mixing zinc salts in the lab always felt slightly intimidating because new formulas meant new reactions. Discovering a compound’s makeup can turn dry theory into something tangible. Zinc acetate dihydrate includes one zinc atom, two acetate groups, and two water molecules. Those two water molecules, known as waters of hydration, help stabilize the compound. They also affect how it dissolves, reacts, and looks. Anyone who handled the powder would see its needle-like crystals—something you’ll remember if you sprinkled too hard or spilled a few grains on your notebook.
Roles Beyond the Beaker
Function comes before flash in the chemical world. Many people know zinc acetate dihydrate as a dietary supplement or an ingredient in lozenges. The zinc here acts as an important micronutrient. The formula ensures enough acetate accompanies zinc so the body can absorb it without discomfort. In the laboratory, researchers count on its purity to run reactions that require a known amount of zinc. Even textile makers use it to transform fibers during production.
Not every formula on a bottle can claim such versatility. Zinc acetate dihydrate also steps in during other chemical syntheses, especially as a source of zinc ions. Kid-friendly science kits sometimes rely on this familiar compound for crystal-growing experiments. All the while, that little ·2H2O tag quietly ensures the substance behaves the way the kit promises.
Safe Use and Reliable Information
Anyone curious about safe handling finds valuable advice from respected resources like the National Institutes of Health, the Centers for Disease Control, or the Material Safety Data Sheets provided in the workplace. These sources highlight safe storage, handling practices, and the benefits and limits of dietary use. Accurate formulas also keep chemists honest: The hydration part isn’t just decorative. Water molecules inside the formula directly shape the way zinc acetate dihydrate interacts in reactions and in the body.
Looking for Solutions
Clear labeling, informed research, and honest reporting help people avoid confusion or unsafe practices. No two hydrates behave the same, so it pays to check the full formula. That little detail can make or break a research result—believe me, adding the wrong version during a university crystallography project throws off measurements and transforms transparent needles into a sticky mess.
Better chemical education stands as a major solution: Teachers who break down formulas like Zn(CH3COO)2·2H2O help build confidence and practical skill. Quality resources, from government sites to reputable encyclopedias, support smarter, safer choices both at the bench and in stores. Good science grows from curiosity, but it relies just as much on small details written right into the formula.
Handling Zinc Acetate Dihydrate in Everyday Labs
Most folks working with zinc acetate dihydrate—whether researchers, teachers, or anyone else dealing with chemicals—have a pretty clear idea of the rules. Yet, mistakes often slip in, either from rushing or from getting too comfortable. This compound shows up as a colorless, crystalline powder that attracts moisture from the air. I’ve seen bottles crust over fast in humid rooms, and that’s when trouble starts. Moisture turns zinc acetate dihydrate clumpy, then it starts to decompose. Purity drops. Anyone relying on accurate measurements or careful chemical reactions ends up frustrated. So smart storage isn’t just bureaucracy—it protects experiments, budgets, and even reputations.
Where You Store Matters More Than Labels Suggest
Temperature makes a big difference. Leave zinc acetate dihydrate in any place above room temperature, and humidity creeps in. I keep mine in a dry, well-ventilated space, away from windows or steam or anywhere folks leave coffee mugs. Labs that run air conditioning in summer or heat in winter sometimes allow pockets of dampness to go unnoticed. I always put the container back on a high shelf, above spills and foot traffic. It keeps accidental splashes, bumps, or temperature fluctuations to a minimum.
Containers: Not All Plastics and Glass Are Equal
Not all containers keep moisture out. I learned this the hard way early in my career, using thin plastic that flexed or didn’t seal tight. Choose tough glass or HDPE bottles with real seals, not just a loose plastic lid. Snap a desiccant pouch inside whenever possible, and swap them out if they get soft or clumped. Stocks of zinc acetate dihydrate store longer with silica gel or molecular sieves protecting them. If the institution shares storage space, add a clear label: date received, date opened, and initials. This keeps anyone borrowing the chemical aware of its age and condition.
Away from Acids and Heat Sources
People sometimes think chemicals with similar names should sit together, but that’s risky. Zinc acetate dihydrate reacts with strong acids or oxidizers. Sparks, hot equipment, or sunlight break it down. One summer, I watched an entire batch go bad just because it sat near a sunny window. Move it away from acids, bases, and strong reducers or oxidizers—cabinets that lock keep it out of the wrong hands, too. Store it as you would anything you don’t want to lose money on or replace mid-project.
Training and Common Sense Outlast Any List of Rules
I trust a colleague or student who pays attention more than one who rattles off rules. Facts support care: the Merck Index and Sigma-Aldrich highlight sealed storage and cool, dry conditions. Chemical safety regulations remind us that loose handling leads to messes, ruined results, and even exposure risks. The CDC notes that improper storage triggers chemical changes, which nobody wants to discover after the fact. Regular checks and open conversations with lab partners help. I suggest a routine sweep—inspect storage once a week. Run through safety data sheets together, especially with new team members. Over time, these habits stick, and zinc acetate dihydrate stays effective, safe, and ready to use.
What Is Zinc Acetate Dihydrate?
Zinc acetate dihydrate sounds like something only a scientist would pick up, but it often appears on product labels. It’s a compound where zinc combines with acetic acid, and it pulls in two water molecules for stability. You might spot it in dietary supplements, some lozenges for colds, or as part of certain pharmaceutical products. Since zinc is an essential mineral, adding it to foods and medications makes sense for health — but not all forms work the same in the body.
Why Zinc Matters for Health
Most people think of vitamin C for fighting colds, but zinc really deserves some of that spotlight. Zinc helps with growth, keeps the immune system working, and supports wound healing. Deficiency doesn’t just weaken immunity — it can stall growth in kids or raise risk for infections. That's why oral zinc supplements get recommended under medical guidance, especially for people with diets low in this mineral.
Understanding Safety: Dose and Source Matter
Many forms of zinc show up in supplements. Zinc acetate dihydrate often gets picked for cold lozenges because it dissolves well and releases zinc ions efficiently. Regulatory agencies like the FDA don’t put zinc acetate on the banned substances list. They allow it in food and dietary supplements, provided companies follow safety limits set for total daily zinc intake.
Problems only start if someone takes too much. The National Institutes of Health (NIH) points to about 40 milligrams of zinc a day as the tolerable upper limit for adults. Too much zinc from any source, including acetate dihydrate, brings risking nausea, stomach cramps, even interfering with copper absorption in your body.
Looking Beyond Supplements
Supplement quality isn’t equal across the board. Not every bottle comes with strong quality control. Taking advice straight from an online forum without talking to a medical professional can lead you down a risky path. Purity matters. Zinc acetate dihydrate used in pharmaceutical and food products must follow strict manufacturing standards to avoid harmful byproducts or contamination.
Real-World Use and Safety Oversight
Doctors sometimes recommend zinc acetate for Wilson’s disease, a disorder in which the body holds onto too much copper. In that case, zinc acetate blocks the gut from absorbing copper, so it saves organs from damage. The prescription version faces FDA scrutiny and quality checks, compared to random internet supplements that may not have reliable sourcing.
The World Health Organization (WHO) recognizes zinc supplementation as vital for populations facing malnutrition, especially children. But they highlight the need for professional monitoring.
Smart Supplement Habits and Solutions
If you need more zinc, food sources like meat, shellfish, dairy, and beans work better for most people. They come packaged with other nutrients and don’t cause excess intake nearly as easily. If you’re reaching for a supplement, read the dosage on the label, watch for possible interactions with other minerals, and look for products certified by trusted third parties. Always tell your doctor if you add a supplement — because underlying health conditions can make even everyday substances risky.
The story for zinc acetate dihydrate boils down to this: it’s safe in regulated, recommended amounts, unsafe when people ignore limits or pick unverified sources. That makes communication with professionals and attention to product labels critical. Real health comes from a mix of good advice, food quality, and smart choices — not just dumping isolated minerals into your daily routine.
A Look at the Real Stuff: Appearance and Feel
Zinc acetate dihydrate arrives as a white, crystalline powder. Anyone who has handled chemical reagents in a lab will spot this sort of powder in bottles labeled for industrial use or university labs. This substance won’t give off much of a scent, and it feels dry and slightly gritty between your fingers—not sticky, not greasy. The crystals break down into finer particles pretty quickly, making it easy to mix into water or other solutions.
The Magic Number: Solubility in Water
One thing I learned early on in chemistry—solubility matters. Zinc acetate dihydrate dissolves in water with hardly any fuss. At room temperature, you can mix about 430 grams of this compound into a liter of water before running into problems. Compare that to salt or sugar, and this is high on the solubility scale. People use that feature in labs and factories for making solutions for plating, pharmaceuticals, and even as a micronutrient in animal feeds. Good solubility means quick mixing and more predictable reactions.
Melting, Decomposing, and Getting it Dry
The stuff melts at around 237°C, but if you keep heating, it doesn’t just liquefy quietly. Instead, it starts to break down, losing its two water molecules. You can watch this dehydration happening—first, the powder starts to clump and the surface turns glassy, and eventually you’re left with anhydrous zinc acetate and water vapor. In industrial processes, controlling temperature keeps things in check, since too much heat generates fumes and alters the results.
Density, Packing, and Handling
Zinc acetate dihydrate weighs in at about 1.74 grams per cubic centimeter, so it’s on the denser side for a powder. This matters most in storage and shipping. You pack it and it settles quickly, so containers don’t end up half-empty after transport. Small, manageable granules make for easy scooping and weighing, which keeps lab mistakes down. It clumps in humid air, though, so proper storage away from moisture and direct sunlight makes a difference—sealed containers keep its properties steady for months.
Why These Physical Traits Matter
Chemists, manufacturers, and even farmers want materials that act predictably. Some powders cake up and turn hard, making them useless. Zinc acetate dihydrate keeps its powdery form under decent storage. Because it dissolves easily and melts at a predictable temperature, factories can automate many processes. Any student or lab worker handling it appreciates that there’s no strong odor or overwhelming health hazards, though as with any lab chemical, gloves and goggles are still smart practice. Mishandling opens up skin irritation or mild toxicity, so common sense wins out here.
Building with Confidence: Supporting Facts
Zinc acetate dihydrate follows patterns we see in other hydrated salts. Research published in the Journal of Chemical & Engineering Data tracks its solubility at varying temperatures, supporting what lab manuals and suppliers list. Industrial safety data sheets highlight its non-combustibility and low volatility—another nod to safer working conditions. By hiring quality control techs and training staff, companies protect both product and people. For every new application—whether nutraceuticals, chemical synthesis, or textile dyes—understanding these physical properties keeps surprises out of the workflow.
| Names | |
| Preferred IUPAC name | zinc diacetate dihydrate |
| Other names |
Zinc(II) acetate dihydrate
Acetic acid, zinc salt, dihydrate Zinc diacetate dihydrate Zinc ethanoate dihydrate |
| Pronunciation | /ˈzɪŋk ˌæsɪteɪt daɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 5970-45-6 |
| Beilstein Reference | 3596618 |
| ChEBI | CHEBI:86441 |
| ChEMBL | CHEMBL251691 |
| ChemSpider | 85711 |
| DrugBank | DB09466 |
| ECHA InfoCard | 03b82799-de7d-41b7-90b7-7a2e334d739e |
| EC Number | 2.7.11.1 |
| Gmelin Reference | 56862 |
| KEGG | C01601 |
| MeSH | D015928 |
| PubChem CID | 57347045 |
| RTECS number | ZL5250000 |
| UNII | TFD68U3P9F |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Zn(C₂H₃O₂)₂·2H₂O |
| Molar mass | 219.50 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.84 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -1.35 |
| Vapor pressure | 0.07 mmHg (20 °C) |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | 9.4 |
| Magnetic susceptibility (χ) | -63.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.422 |
| Dipole moment | 1.27 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1034.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1506.2 kJ/mol |
| Pharmacology | |
| ATC code | A12CB01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 Oral Rat 2,824 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 2,820 mg/kg |
| NIOSH | SR2740000 |
| PEL (Permissible) | PEL: 15 mg/m³ |
| REL (Recommended) | 30 mg of Zinc per day |
| Related compounds | |
| Related compounds |
Zinc acetate
Zinc sulfate Zinc chloride Zinc oxide Zinc nitrate Zinc gluconate |



