Uranyl Acetate: A Practical Look at Its Role and Challenges
Historical Development
Uranyl acetate traces its roots back to the rapid growth of chemistry in the nineteenth century. As researchers explored uranium’s complex chemistry, they discovered this compound by combining uranium salts with acetic acid. Chemists in the early 1800s relied on it for coloring glass and ceramics, drawn to the vivid yellow-green tint. For much of the twentieth century, uranyl acetate carved out a specialized niche in microscopy and analytical chemistry. Its popularity grew alongside advances in electron microscopy after World War II. Stains based on uranyl acetate revolutionized cell biology, making cell membranes and organelles stand out under high magnification. By the 1980s, nearly every electron microscope user counted on uranyl acetate to push beyond previous imaging limits. Over decades, safety standards evolved as scientists learned more about toxicity, but the demand for this chemical in high-resolution imaging never waned.
Product Overview
Uranyl acetate shows up as a striking yellow-to-green crystalline powder. Laboratories want it in both powder and solution forms, catering to the needs of electron microscopy, analytical chemistry, and nuclear science. Companies usually ship it in airtight amber glass to protect from moisture and light. Some suppliers offer uranyl acetate ready to use in aqueous or alcohol-based solutions. That saves time in labs focused on routine imaging. With strict nuclear regulatory oversight, reputable sources always provide certificates of analysis and detailed batch tracing.
Physical & Chemical Properties
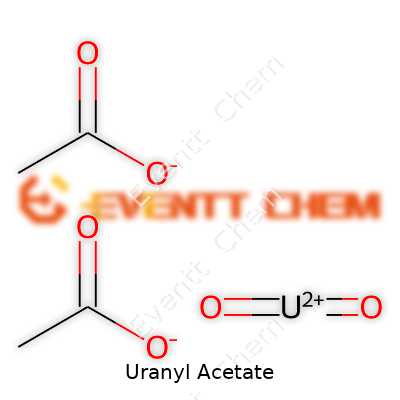
Uranyl acetate (UO2(CH3COO)2·2H2O) features a molecular weight around 424 g/mol. The dihydrate version appears yellow-green and dissolves easily in water, ethanol, and acetone. The solution takes on a greenish tinge, which experienced microscopists instantly recognize. The acid reacts promptly with organic bases, breaking down above 300°C; released uranium oxides produce a marked green flame. High atomic mass and electron-dense uranium atoms sit at the core of uranyl acetate’s property set, enabling electron micrographs to reach deep contrast. In the solid form, the crystals pack tightly, and even a small quantity goes a long way when preparing staining solutions.
Technical Specifications & Labeling
Each shipment of uranyl acetate arrives with labeling in line with international transport codes for radioactive material. Purity standards usually hit 99% and above, with rare earth and heavy metal contaminant levels spelled out in technical data sheets. Certified reference material guarantees reliability for regulated applications in research and diagnostics. Markings always state shelf-life, batch number, storage temperature, and handling precautions. Radioactivity limits generally meet exemption thresholds for most lab users, but documentation—safety data sheets, isotopic purity certificates, and transport compliance—is required for every move from supplier to end-user.
Preparation Method
Preparing uranyl acetate means dissolving measured amounts in distilled water or alcohol, heating gently, and mixing until every crystal vanishes. Staff performing the work rely on chemical-resistant gloves and ventilation. Accuracy here makes all the difference for imaging consistency. After dissolving, labs filter to eliminate any undissolved specks—those can muddy microscope images. Some protocols demand freshly prepared solutions for each staining session to minimize the risk of reduced uranium or hydrolysis products.
Chemical Reactions & Modifications
Uranyl acetate reacts as both an oxidizer and an acid. Adding alkali displaces acetate, forming yellow or green uranium peroxides or hydroxides. In the lab, uranyl acetate joins with phosphate-rich tissue, which forms dark, stable deposits under an electron beam. Organic transformations remain rare—nobody wants accidental release of free uranium. Heat, light, and pH changes can convert uranyl acetate to less soluble forms, which some labs observe as dropped-out precipitate in aged solutions. Researchers sometimes try to modify the molecule for better selectivity or lower toxicity, but uranium’s heavy atom makes most derivatives expensive to test and difficult to handle outside of specialized facilities.
Synonyms & Product Names
Uranyl acetate travels under several names: Uranium diacetate, uranyl ethanoate, and simply UA among microscopy users. Product codes—sometimes including CAS number 541-09-3—appear on supplier labels. Some old texts refer to it by German names like Uranylessigsaure, especially in the context of European glassmaking and early inorganic chemistry research. Commercial sources occasionally market stabilized or low-dust versions to reduce handling risks, but the core substance stays the same.
Safety & Operational Standards
Accepting uranyl acetate into any laboratory demands a real commitment to safety. Uranium’s radioactivity creates obvious regulations, but chemical toxicity poses even more immediate risks. Users work within fume hoods, with gloves and eye protection standard. Any spill means an immediate cleanup: wet wipes, chelating solutions, and special disposal routes. Staff keep exposure logs, especially in institutions with annual radiation audits. For transport, rules specify type-tested packaging, secure documentation, and transport on designated routes only. Waste leaves the building through approved nuclear waste handlers, not down the drain. Some longtime researchers recall when safety protocols were looser, but today, one lapse can jeopardize a whole lab’s compliance record.
Application Area
Uranyl acetate shines in transmission electron microscopy (TEM). Pathologists, botanists, and micro-engineers use it to add electron density to biological samples, amplifying detail down to the nanometer scale. When tracing virus particles in tissue, or mapping kidney membrane thickening, the contrast from uranyl acetate unveils structures that else blend into the background. Beyond imaging, some analytic chemists reach for uranyl acetate as a selective precipitating or titrating agent. Nuclear fuel researchers sometimes explore its solution chemistry, seeking better ways to recycle spent uranium or safely extract uranium from ore.
Research & Development
Today’s research often explores safer staining alternatives or ways to strip uranium out of laboratories altogether. New stains based on rare-earth metals or non-toxic organic molecules offer comparable contrast in TEM without uranium’s drawbacks. Some researchers have made headway with lanthanide-based solutions that match uranyl acetate for imaging, but these often cost more or deliver poorer shelf stability. Instrument makers keep pushing detector resolution, hoping to make ultra-high-contrast stains less critical. Meanwhile, green chemistry advocates work on novel routes to break down uranyl acetate waste, using ion exchange, bioremediation, or advanced precipitation techniques. Funding bodies often favor projects that cut uranium use and develop standards for residue monitoring in lab wastewater.
Toxicity Research
Uranium compounds like uranyl acetate bring a web of toxicity hazards. Inhalation or ingestion can harm the kidneys, where uranium concentrates during filtration. Animal studies show DNA damage and interference with cell division at high doses. Uranium’s weak radioactivity poses less threat than its ability to disrupt metabolism and induce organ stress even at low exposure. Chronic lab exposure calls for routine testing, training, and environmental monitoring. Several large epidemiological studies in nuclear industry workers failed to find significant cancer risk at low doses but spotlighted chemical nephrotoxicity. Waste disposal standards keep tightening each decade, shaped by research from toxicology labs and international watchdogs such as the International Atomic Energy Agency and US Environmental Protection Agency.
Future Prospects
Looking ahead, stricter regulation and growing environmental awareness push users to phase out uranyl acetate from routine staining and analysis. Research labs now experiment with digital image enhancement, seeking to wring more detail from safer stains or even unstained samples. If green chemistry advances keep pace, fully biodegradable stains may become the norm for biology labs. Uranium recovery and recycling could reduce waste for industries that still need high-precision uranium chemistry. Until substitutes become fully reliable and affordable, uranyl acetate will stick around for cutting-edge research and critical diagnostic imaging. But each year, more labs join the move toward a low-toxicity, no-radioactivity future in analytical science.
Looking Beyond the Chemical Name
Uranyl acetate isn’t something you find on a pharmacy shelf, and most folks outside of research labs might not even hear about it. Yet, for researchers in biology and material science, this yellow powder plays a bigger role than most realize. I remember my first time in a transmission electron microscopy (TEM) lab; the tiny bottle marked with radiation warnings felt intimidating. No one passed around those bottles casually. Every interaction with uranyl acetate felt serious, but people kept using it. There’s a good reason for that.
The Heart of Electron Microscopy
Electron microscopes let researchers peek inside cells, viruses, and nanoparticles. Without clear detail, a TEM image is just a mess of shadows. Uranyl acetate fixes that problem. Sitting at the microscope, you take wafer-thin slices of a sample—anything from plant cells to bits of brain tissue. Uranyl acetate binds to specific biological structures and gives much better contrast in the final images. Protein and membrane details jump out in sharp black and white. Every big discovery you’ve seen from inside a human cell probably relied on staining with this compound.
Why Researchers Can’t Just Swap It Out
Labs have experimented with alternatives, hoping to dodge the radioactive tag tied to uranium’s reputation. Lead citrate entered the scene as a possible replacement, but it just doesn’t bring the same detail, especially for certain cellular parts. Digital manipulation and new AI-driven image techniques can’t fill that gap, at least not yet.
Risks You Can't Ignore
Uranyl acetate is radioactive. Not in the splitting atoms, glowing-green kind of way, but everyone takes precautions. In one lab I visited, there was a dedicated waste bin for used paper towels and gloves. Anyone handling the powder wore gloves and sometimes goggles, especially in poorly ventilated rooms. Skin contact brings long-term risks, and inhaling dust poses even bigger dangers. Rules about its use are strict and disposal can get expensive. Still, its effectiveness means labs keep ordering it, since the science often outweighs the hassle.
Possible Paths Forward
With stricter global rules about uranium, many labs have started scanning research papers for safer staining options. Some researchers play around with lanthanide-based stains, which show promise for lower toxicity. Others test digital enhancement tools to boost the contrast of ordinary stains. The path won’t be simple—even with funding and technical know-how, a new standard takes years to adopt. In the meantime, strict lab safety, proper training, and careful waste handling make it possible to keep using uranyl acetate without inviting extra risk.
Bottom line: Uranyl acetate gives researchers powerful detail they can’t get other ways. The pressure to find safer substitutes means we may see new breakthroughs soon, but for now, most cell biologists and microscopists keep it close at hand, stripes of caution tape and all.The Story Behind Uranyl Acetate in the Lab
Uranyl acetate lives on the supply shelves of thousands of research labs. This yellow-green compound helps scientists stain tissue for electron microscopy. Some turn nervous when they hear that “uranyl” part—it comes from uranium. It carries a warning on every bottle. Yet most folks probably never step back to ask, “Is this stuff actually radioactive, or just toxic?”
Real Danger Lies in Toxicity
In my years sweating over a lab bench, nobody ever buzzed with radiation anxiety around uranyl acetate. Instead, every bottle sat behind a locked cabinet, away from snacks and water bottles. Researchers wore gloves and worked with care, less out of fear of radiation, more because of its heavy metal nature. Here’s the reality: uranyl acetate contains uranium, but not the kind worried about in nuclear reactors or weapons. Uranium-238, the dominant isotope, has such a long half-life that it barely emits radiation. Set a Geiger counter beside it and the readings barely flicker above natural background levels.
Its bigger risk comes from chemical toxicity. Uranium attacks the kidneys. Inhaling the dust or letting it sit on skin can bring harm, as with other heavy metals like lead or mercury. Ingesting even tiny bits could become a problem over time. Safety rules for this chemical line up with those for other heavy metals: gloves, goggles, good ventilation, and separate trash. Eating lunch or sipping coffee nearby becomes a bad idea.
Radioactive by Nature, Safe by Practice
The question of radioactivity often drifts through the conversation. Many expect glowing green and Geiger counters gone wild. That’s Hollywood. The uranium in uranyl acetate sheds a trickle of alpha and beta particles—energy most masks, gloves, or even paper block easily. Still, no one treats it lightly. Some countries send it through extra government paperwork because of its atomic nameplate, though it doesn’t behave like nuclear-grade material.
When I first handled it, I asked the old hands in the lab what concerned them most. They pointed at a poster above the sink showing kidney diagrams, not one mentioning radiation burns. Disposal of uranyl acetate waste always meant filling out forms and boxing it as hazardous chemical, not radioactive. Safety courses focused on chemical exposure, not dosimeter badges or radiation suits.
What Labs Should Do Next
Safer alternatives do exist. Lead citrate, for instance, also helps enhance contrast in microscopy but brings its own set of risks. Some push for newer, non-toxic stains. The problem: these rarely match uranyl acetate’s clarity under the microscope. For now, many researchers accept the balancing act—using proven safety habits, securing their chemicals, and respecting the risks in front of them.
Education makes the biggest difference. New lab workers need to hear the real stories—the kidney risks, the forbidden sandwich in the electron microscopy suite, the careful taping up of every waste bottle. Gloves, gowns, fume hoods, and smart labeling do more than regulations alone can achieve. When treated with respect and understanding, uranyl acetate serves science well, without drama, glowing or otherwise.
Moving Toward Transparency and Alternatives
Staying transparent—posting clear safety data where people can read it and updating protocols—helps keep everyone in the know. Funding more research into safer, reliable stains could give the next generation of scientists new tools for discovery. Until then, trust the facts, remember what makes uranyl acetate hazardous, and don’t lose focus on personal protection and smart lab practice.
A Practical Perspective on a Dangerous Chemical
Uranyl acetate doesn’t get much attention outside labs. Yet, this yellow-green powder can spell real trouble if not given respect. I remember walking into a storage room in grad school, humming overhead lights and all, and spotting an unlabelled glass bottle. The lab manager checked it, shook her head, and pulled everyone aside: “This could hurt you badly.” Nobody there forgot that message.
Why Proper Storage Matters
Uranyl acetate holds a double punch: it’s both radioactive and toxic. Skin contact or inhalation can cause problems most folks never see in a chemistry class. According to the CDC, inhaled dust from uranyl acetate damages kidneys, and the alpha radiation may up long-term cancer risks. Correct storage goes way beyond ticking off OSHA requirements—it safeguards health, supports honest science, and also limits environmental harm.
Finding the Right Spot
Not every shelf can handle this stuff. The ideal home for uranyl acetate: a sturdy, lockable, acid-resistant cabinet, clearly marked with signage that warns about both toxicity and radioactivity. These signs have to stand out so even after a long shift, nobody misses the risks. I’ve seen accidents where mislabeled chemicals led to dangerous mix-ups. Mark those containers persistently and update logs every time something moves in or out.
Humidity turns uranyl acetate clumpy, sometimes speeding up container corrosion. Dry conditions with steady room temperature keep it stable. Big temperature swings crack containers, especially those made from glass. Forget storing near anything organic or flammable. Uranyl acetate can react with common solvents or acids, creating fumes or worse. Some protocols separate it from food, drinks, and break areas, since small particles can hitch a ride on dust. This isn't about paranoia—it’s honest caution from every chemist who’s had to clean a spill.
Thinking About Security
Security adds another layer. Uranyl acetate isn’t just dangerous in a health sense; it interests people for the wrong reasons. Lab managers restrict access to authorized users, logging who enters, especially in shared campus spaces. The Nuclear Regulatory Commission expects real accountability. Strangers and students shouldn’t even get close.
Prepping for Accidents
Emergencies test every safety plan. Spill kits, gloves, lab coats, and safety goggles can’t be crammed in the next room. They stay within arm’s reach. Training drills remind workers what to do if there’s a splash or dust release; confusion during a real event causes injuries. Disposal matters, too—waste containers for uranyl acetate aren’t mixed with everyday trash. They get treated by professionals. Mishaps, even small ones, cost real money and time.
Improving Safety Beyond Labels
I’ve met researchers who set up QR codes on cabinets with instructions and emergency numbers. Others introduced weekly checklists to verify everything stays as it should. A culture of asking questions—“Who moved the bottle?” or “Does the lock still work?”—catches small problems before they get big.
Careful storage slows down work a bit, but nobody regrets those extra seconds. Lab stories make one thing clear: cutting corners here can change lives for the worse. Respecting uranyl acetate means clear labeling, dry cabinets, strict accountability, and always knowing who’s handling the bottle.
Why Extra Care Matters
Uranyl acetate shows up a lot in electron microscopy labs. Its ability to stain biological samples means it's valuable for researchers, but it also brings significant risks. Uranyl acetate contains uranium, making it both toxic and radioactive. Even brief, careless handling leaves workers open to real health problems—both right away and years down the road.
Clear Risks, Real Stakes
My first encounter with uranyl acetate brought a safety seminar and plenty of warnings. The instructor didn’t sugarcoat anything: exposure can harm the kidneys and liver, contaminate the workspace, and even present a cancer risk with sustained contact. The radioactive component isn’t as strong as something in a nuclear reactor, but it still leaves a mark on those who ignore basic precautions. Inhaling powder dust, spilling liquid on your skin, or letting it splash into your eyes can create a medical emergency. Long-term risk sneaks up on people who take shortcuts.
The Right Gear
The first line of protection is personal gear. Researchers learn quickly to trust in good gloves, safety goggles, and a long-sleeved lab coat. Nitrile gloves stand up better than latex, especially if any spills happen. Splashes love to bounce into unexpected places, so wraparound goggles keep eyes safe. I always check that my coat fits tight on my wrists, leaving nothing exposed.
Smart Workspace Habits
Ventilation isn’t optional. Labs working with uranyl acetate use certified chemical fume hoods. That airflow cuts down on dust and fumes before they have time to settle. Solid technique matters as much as the machines that back it up. Measuring and transferring powders with a minimum of force keeps dust down, and every container stays closed unless in use. No eating, drinking, or casual snacking ever happens in these spaces. I wipe down the counters with a fresh disposable towel and toss it in designated radioactive waste bins. That small effort pays off every time.
Clean-Up and Disposal
Any waste—powder, solutions, wipes, or broken glass—goes into specially labeled containers. Cleaning up after a session means double-bagged, tagged, and logged for the radiation safety team. Liquids don’t go down the drain. Even after the last step, hands see thorough washing with soap and warm water. I learned to log every gram used, and to scan for surface contamination with a handheld Geiger counter. Scrubbing lab benches and washing gloved hands before removing any equipment helps prevent accidental spread.
Training—No Substitute
Workers new to uranyl acetate must complete in-person training and show understanding before opening a single vial. Each person passes a safety quiz and reviews scenario drills for spills or accidental contact. This isn’t bureaucracy—it fills in the gaps that a few written instructions can miss. People, after all, forget steps and rush under deadlines. Practice and repetition keep the good habits strong.
Health Tracking
Many workplaces keep tabs on long-time users with regular urinalysis and check-ups. Early signs of heavy metal exposure show up in kidney or liver changes before symptoms get serious. It’s not just about catching illness, but helping employees work longer without worry. I’ve seen coworkers take comfort knowing their health gets honest attention if something slips through protective barriers.
Take Precautions Seriously
No research is worth risking health. I remind myself and colleagues before each project: every shortcut gives up something more important. A culture that prizes safety won’t slow down research—it makes sure everyone comes back for the next experiment.
Understanding Uranyl Acetate’s Place in Science
Uranyl acetate shows up in labs around the world. It plays a crucial role in staining biological samples for electron microscopy. I remember using it during a long week in grad school, squinting at images of mitochondria so I could catch the smallest change in cell structure. Those salts turned invisible membranes into striking contrasts that told stories about health and disease. Anyone who’s worked with it knows how vital it can be for research.
Uranyl Acetate and International Regulation
Flying with a tube of this stuff isn’t like shipping a textbook or a box of beakers. At the heart of it is uranium, a word that raises eyebrows far outside the world of electron microscopes. Governments consider uranyl acetate a radioactive substance. Even though its radioactivity falls on the low end, rules around uranium compounds stay tight, largely thanks to concerns about nuclear proliferation and safety. Try convincing a customs agent at Heathrow or JFK that this yellow bottle doesn’t belong in a power plant.
Shipping rules boil down to national and international agreements. Across the United States, uranyl acetate falls under licensed handling. The Nuclear Regulatory Commission stepped in back in 2023, making distributors and labs jump through extra hoops to prove the chemical won’t end up somewhere dangerous. In the European Union, the legislation looks just as strict. Certain countries have given up on importing uranyl salts altogether, and others only allow trusted labs with heaps of paperwork and oversight to bring it in. Australia and Canada share similar stories.
Why This Should Matter Beyond the Lab
Some might see this as just a scientist’s headache. It’s more than that. Delays in shipping or outright bans can slow down breakthroughs in medicine, plant science, and environmental monitoring. Researchers shared warnings last year that electron microscopy work would grind to a halt in some regions without a reliable supply of this compound. Students end up staring at blurry images, and ongoing studies stall. This isn’t about one shipment. Scientific progress depends on labs being able to share and receive tools without unnecessary bottlenecks.
Risk, Reputation, and Possible Paths Forward
No one can argue about the need for proper oversight on radioactive materials. A few grams of uranyl acetate might seem harmless tucked in a science classroom, but scale it up or send it without controls, and the consequences turn serious. At the same time, not every overseas order comes with risk. Trusted academic and government labs offer decades of secure handling. Maybe regulators could set clearer, risk-based guidelines, instead of blanket restrictions that punish responsible teams. Stronger digital tracking can trace each order, adding a layer of oversight without bringing the whole system to a crawl.
Some scientists have started testing alternative stains to bypass the issue entirely. Results so far have been mixed. Alternatives may not deliver the same image quality or stability. For now, finding a way to responsibly and transparently ship uranyl acetate stays important for the future of basic and biomedical research worldwide.
| Names | |
| Preferred IUPAC name | diacetatodioxouranium |
| Other names |
Acetic acid, uranyl salt
Uranyl diacetate Uranyl(2+) diacetate Uranyl ethanoate Uranyl(II) acetate |
| Pronunciation | /ˈjʊə.rə.nɪl əˈsiː.teɪt/ |
| Identifiers | |
| CAS Number | 541-09-3 |
| Beilstein Reference | 87376 |
| ChEBI | CHEBI:86359 |
| ChEMBL | CHEMBL4295392 |
| ChemSpider | 54674 |
| DrugBank | DB14075 |
| ECHA InfoCard | 100.008.170 |
| EC Number | 200-598-5 |
| Gmelin Reference | 7167 |
| KEGG | C18885 |
| MeSH | D020081 |
| PubChem CID | 2724376 |
| RTECS number | YQ9625000 |
| UNII | A1O7W7M817 |
| UN number | UN2910 |
| Properties | |
| Chemical formula | UO2(C2H3O2)2 |
| Molar mass | 424.13 g/mol |
| Appearance | Pale yellow-green crystalline powder |
| Odor | Odorless |
| Density | 3.608 g/cm³ |
| Solubility in water | Very soluble |
| log P | -2.97 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.2 |
| Basicity (pKb) | 6.1 |
| Magnetic susceptibility (χ) | -43.0e-6 cm³/mol |
| Refractive index (nD) | 1.598 |
| Dipole moment | 2.36 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1460 kJ/mol |
| Pharmacology | |
| ATC code | V10XA02 |
| Hazards | |
| Main hazards | Toxic if swallowed, causes severe skin burns and eye damage, may cause genetic defects, suspected of causing cancer, very toxic to aquatic life. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H350, H360Df, H372, H301, H332, H411 |
| Precautionary statements | P201, P202, P260, P264, P270, P273, P280, P284, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P314, P320, P330, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-0-Radioactive |
| Lethal dose or concentration | LD₅₀ oral (rat): 204 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 204 mg/kg |
| NIOSH | YD0350000 |
| PEL (Permissible) | 0.2 mg/m3 |
| REL (Recommended) | 0.003 mg/m³ |
| IDLH (Immediate danger) | 5 mg U/m³ |
| Related compounds | |
| Related compounds |
Uranium tetrachloride
Uranyl nitrate Uranium(IV) oxide Uranium(VI) oxide |



