Thionyl Chloride: A Grounded Look at Its Journey, Uses, and Hazards
Historical Development
Thionyl chloride has been circulating in the chemical industry since the late 19th century. Chemists found it incredibly useful as a reagent almost right after its first synthesis, which took place as industry and research moved into the era of more complex organics and pharmaceuticals. Unlike many chemicals that faded with new discoveries, thionyl chloride stayed relevant. Chemical production boomed in the 20th century, and this compound became a mainstay. Most organic labs and industrial plants dealing with advanced chemistry featured some thionyl chloride at a certain point. The need to efficiently convert alcohols to chlorides made thionyl chloride a stand-out choice for researchers, and as chemical manufacturing scaled up, so did its production. From early dyes to modern medicine, demand only grew.
Product Overview
Thionyl chloride, known scientifically as SOCl2, packs quite a punch for such a tiny molecule. Its ability to act as a chlorinating agent makes it a workhorse for both small-scale research and heavy industry. What makes thionyl chloride so essential? Where other chlorinating agents involve complicated set-ups or harsh conditions, this liquid offers high yields with less mess. It helps bring pharmaceutical compounds up to spec, and chemical manufacturers use it when turning out agrochemical intermediates and dyes. No wonder just about any industrial chemist has run across SOCl2 in some form or another.
Physical & Chemical Properties
At room temperature, thionyl chloride shows up as a clear to slightly yellow liquid, easy to spot thanks to its sharp, biting odor. It comes with a boiling point close to 79°C and a melting point at -104.5°C. With a density of about 1.64 g/cm3, it sinks into just about any organic liquid. Thionyl chloride reacts fiercely with water, releasing hydrochloric acid and sulfur dioxide – both irritants that can cause trouble fast if spilled. These properties demand good ventilation and careful handling in the lab or plant. Its volatility helps in reactions, but it’s quick to release hazardous fumes if containers leak.
Technical Specifications & Labeling
Thionyl chloride typically gets bottled and shipped in steel drums or glass containers with robust seals. Labels spell out the corrosive nature, warning handlers about serious risks like chemical burns and toxic vapors. In my experience, quality control teams check product purity (usually above 99%) using gas chromatography and look out for impurities like sulfur dioxide, which signals breakdown or poor manufacturing practice. Real-world containers throw caution signs in bright colors, and shipping regulations require strict records for where, how, and to whom this compound is going. Documentation from suppliers includes batch numbers and recommended storage temperatures, and anyone receiving a shipment deals with a clear set of handling rules before they ever pop a cap.
Preparation Method
Most thionyl chloride comes from treating sulfur dioxide with phosphorus trichloride at moderate temperatures. Large-scale operations focus on minimizing side reactions that put out unwanted byproducts like dichlorosulfoxide or elemental chlorine. Proper process design cuts down on waste and makes recycling reagents possible. Years ago, small labs made their own SOCl2 using variants of that process, but tight environmental rules led to consolidation into major facilities that offer better containment and scrubbing systems. Production lines tighten up on leaks, and even small emissions get scrubbed out before they reach the environment.
Chemical Reactions & Modifications
Thionyl chloride’s best-known reaction is turning alcohols into alkyl chlorides. It replaces the hydroxyl group with a chloride and, because SOCl2 is a liquid, it leaves no solid wastes if done right. What drew me to this chemical during early lab days was its clean byproducts – sulfur dioxide and hydrochloric acid, both gases that are easy to remove or neutralize. This makes for quick purifications, which anyone who’s spent hours cleaning up byproducts can appreciate. Thionyl chloride also stands out for making acid chlorides from carboxylic acids, which open the door to a slew of other transformations – esters, amides, and more. Chemists sometimes modify SOCl2 through reactions to tweak selectivity or to attach it to a polymer backbone for special applications in battery technology.
Synonyms & Product Names
Thionyl chloride appears in catalogs and labs under several names. Some bottles read 'Sulfinyl chloride.' Others get more technical: 'Sulfurous dichloride.' Chemists just call it SOCl2 for short. Industrial brands may label it as 'chlorinating agent SC,' or even use trade names if part of a proprietary blend. Knowing synonyms avoids confusion, since many industries swap between names on drawings, safety documentation, and procurement orders. It always pays to check chemical formulas, not just product names, before loading up a reaction flask or receiving a shipment.
Safety & Operational Standards
Handling SOCl2 takes respect and planning. Even with the right gloves and goggles, inhaling vapors or letting it touch skin leaves lasting harm. It hydrolyzes fast with even humid air, so containers must stay tightly closed, and fume hoods get fired up before weighing or transferring the liquid. Emergency showers and eyewash stations get checked weekly in labs where thionyl chloride shows up, and disposal involves neutralizing any leftover material with sodium carbonate in ice-cooled setups to prevent runaway reactions. Regulatory bodies like OSHA in the US and ECHA in the EU spell out workplace exposure limits, while local rules set even tougher standards for storage and spill response. Experienced workers double up on checks before opening up containers, and any sign of a leak triggers a snowball of incident response, not a casual clean-up.
Application Area
Thionyl chloride turns up everywhere that complex chlorinated compounds matter. In the pharmaceutical world, it steps in when making antibiotics, anti-inflammatory drugs, and even new COVID-19 treatments. Agrochemical makers rely on it to tweak pesticides for higher crops yields and stronger resistance to bugs and mold. Dye manufacturers use SOCl2 to craft vivid colors that don’t wash away or fade in sunlight. Electronics industries lean on thionyl chloride–based lithium batteries, banking on its high-energy density for long-lasting power in sensors, military gear, and remote medical devices. Without thionyl chloride, the pace of new development across all these sectors would slow down, and alternative routes often cost more and produce more waste.
Research & Development
Chemists around the world keep hunting for new ways to use SOCl2 that increase yield or selectivity while cutting down on the nasty leftovers. Research teams regularly publish improvements on catalyst selection, solvent effects, and energy inputs in journals like the Journal of Organic Chemistry. Years of trial and error led to tandem reactions involving thionyl chloride that cut steps out of multi-stage syntheses, letting companies reach complex targets faster and with less waste. Computational chemists build models that predict reaction outcomes with SOCl2, reducing the time spent on frustrating pilot-scale failures. Academic labs train graduate students on SOCl2 as a rite of passage, teaching both creativity and caution, since a slip-up teaches respect in a hurry.
Toxicity Research
Toxicologists dug deep on how thionyl chloride affects the human body. Brief exposure triggers coughing, chest pain, and shortness of breath. At higher concentrations, risks jump to chemical burns, lung damage, and serious injury to eyes or skin. Several studies featured in 'Toxicology Letters' highlighted how repeated exposure, even at low levels, increases risks for chronic bronchitis and contact dermatitis. Long-term environmental studies show that accidental releases into air or water kill aquatic life due to rapid acidification, which can spread downstream fast. Whenever health and environmental agencies review chemical incident reports, SOCl2 pops up as a major cause for concern, and outbreak simulations keep hospitals on guard for sudden environmental releases.
Future Prospects
Companies and researchers look to cut thionyl chloride’s environmental and health drawbacks, searching for substitutes or greener methods wherever possible. Bio-based chlorination pathways see ongoing development, with promising results that still can’t match SOCl2’s efficiency and yield. In lithium battery production, teams work on solvents and electrolyte formulations that sidestep thionyl chloride, but cost and reliability gaps slow adoption. Foolproof containment, automatic leak detection, and system-level monitoring are newer safety upgrades that show promise for bigger plants. Governments push harder for emergency planning and stricter emissions controls, and large buyers focus on suppliers with strong stewardship programs rather than price alone. While chemists seek ways to recycle or neutralize SOCl2 byproducts in-process, total phase-out looks unlikely for the foreseeable future. Its track record for reliability and efficiency keeps it locked in, but new tools and smarter procedures show the path forward may balance safety with industry needs better than ever before.
How Thionyl Chloride Shapes Modern Industry
Walk past any chemistry lab and the sharp scent that might tickle your nose probably comes from thionyl chloride. This chemical doesn’t grab headlines, but it plays a huge role in putting modern science into action. Thionyl chloride appears as a volatile, colorless-to-pale yellow liquid with a reputation—both for its usefulness and for the need to handle it with care. My own experience handling chemicals started in a university lab where the instructor treated the bottle of thionyl chloride like a rare gem. That early exposure taught me the respect it deserves and why people in pharmaceuticals, batteries, and electronics reach for it.
Behind the Scenes in Pharmaceuticals
Most medicines on the pharmacy shelf owe their existence to precise chemistry, and thionyl chloride acts as a behind-the-scenes helper. Drug makers rely on it to transform acids into acyl chlorides—a crucial step that lets them create new compounds and, eventually, new therapies. Antibiotics, pain relievers, and cancer treatments sometimes need thionyl chloride somewhere in their family tree. This role matters because one misstep, and the whole batch gets poured down the drain. Having a tried-and-true reagent like thionyl chloride shaves off both risk and wasted effort.
Batteries for a Connected World
Open up a piece of industrial equipment, or pace around with your phone, and you’re probably carrying the results of battery chemistry that uses thionyl chloride. Primary lithium batteries—long lasting and quick to deliver bursts of energy—often rely on lithium thionyl chloride cells. These batteries power gas meters, alarms, remote sensors, and tracking devices, working silently for years without a recharge. If you’re reading a utility meter outside your house or checking wireless sensors in distant locations, chances are those devices depend on thionyl chloride’s knack for storing and releasing energy reliably even at low temperatures.
Electronics and Everyday Tools
Chip makers and electronics engineers can’t do their job without a way to purify and etch silicon. Thionyl chloride stands out as one of those workhorses in circuit fabrication. It cleans surfaces and prepares semiconductors for the precise dance of adding microscopic pathways and switches. Without it, electronics manufacturing would slow down or cost more, and progress in the digital world would hit speedbumps.
Challenges and Responsible Handling
Every tool has its dangers, and thionyl chloride is no exception. Anyone who’s ever splashed a drop or caught a whiff in the lab knows its strong, acrid fumes mean business. Contact with water sends it fizzing into hydrogen chloride gas, so storage and handling practices must be airtight. Spills require immediate attention. Governments and companies mark strict rules around its use because accidents can hurt both workers and the environment.
Looking for Safer Approaches
Research never stands still. Chemists keep searching for greener alternatives or safer conditions where thionyl chloride is used. Some labs now experiment with less toxic reagents or sealed processing units that trap fumes. Others design new reactions that achieve the same transformation—making an acyl chloride or powering a battery—without using such a harsh chemical. The pressure to find safer solutions isn’t just a matter of preference. It’s about protecting health, lowering costs from accidents or spills, and cutting down on the environmental footprint of science and industry.
A Chemical Not to Underestimate
Some chemicals leave a lasting impression the first time you come across them in the lab. Thionyl chloride fits this category. It’s sharp on the nose, and you’ll find out fast that this clear, colorless liquid doesn’t mess around. Chemists and workers handling this compound have every reason to treat it with respect. The risks are real: burns on skin, eye damage, dangerous vapors, and gas release the second it hits water. There’s a short list of chemicals where the right safety steps make the difference between a routine day and a trip to the emergency room, and thionyl chloride sits at the top.
The Basics: Hands, Eyes, Lungs
A well-fitted pair of chemical-resistant gloves (think Viton or butyl rubber—not your regular latex) keeps hands protected. Safety goggles, face shields, and full coverage lab coats are not optional. Lucky enough to have a fume hood? Always use it. Thionyl chloride releases hydrogen chloride and sulfur dioxide if it’s even close to water—and that includes water in the air on humid days. Both gases can choke you and leave lingering lung issues. Inhaling them during a spill changes a regular shift into a nightmare. Respirators with acid gas cartridges offer critical backup if things go sideways. Folks in the industry know: you don’t take even a tiny shortcut here.
Ventilation and Environment Matter
I remember walking into a workspace where thionyl chloride got stored in a sunlit spot. That heat made the vapors worse and put everyone at risk. This compound belongs in a cool, dry chemical storage cabinet, far from anything containing water—including fire suppression systems. Spills need special pads and neutralizers like sodium bicarbonate or soda ash, never water or anything resembling water. Without proper clean-up tools close at hand, response time drags, and the danger grows.
Training and Real-World Readiness
A lot of industries stress safety, but working with thionyl chloride, you discover that knowledge alone isn’t enough. Untrained staff can cause trouble—mismatching storage containers, skipping gloves, or misunderstanding what “dry area” actually means. Regular training and drills build habits. In my own experience, tabletop exercises where teams practice spill response cut down on panic when the real thing happens. Every person in the area should know the evacuation route and how to use the eyewash and emergency shower. No one thinks they’ll need it, right up until they do.
Down the Road: Safer Alternatives and Better Planning
Thionyl chloride has specialty uses—making pharmaceuticals, pesticides, batteries, and advanced materials. But some labs and factories look for workarounds or safer substitutes. Phosphorus oxychloride might replace it in some cases, yet that brings its own baggage. Real progress comes from process improvements and automation that limit human exposure. Routine reviews of storage, ventilation, and automation systems shake out risks before someone pays the price.
Keeping a Healthy Respect
Those who have handled thionyl chloride know it demands constant respect. Safety relies on strong habits, solid personal protective equipment, and equipment that works as hard as you do. Anyone new should shadow experienced staff and ask questions freely. Mistakes—and complacency—cause more harm than ignorance. It only takes one rushed moment or distraction in a busy shift to set off a chain reaction you won’t forget. Safety isn’t just procedure; it’s attitude. That keeps thionyl chloride from turning from a tool into a tragedy.
Chemistry in Real Life
Thionyl chloride carries the formula SOCl2. That three-part combination—sulfur, oxygen, and chlorine—shows up in labs through a colorless liquid with a strong, choking odor. Most chemists remember its reputation before its formula. The fumes alone make lasting memories. But no matter how you remember it, that simple combination powers many processes in research, manufacturing, and even at home, hiding beneath layers of daily convenience.
How SOCl2 Shapes Science and Industry
Synthesizing chemicals often starts with materials most would never recognize. Turning carboxylic acids into acyl chlorides, for example, can feel frustrating and tedious. SOCl2 transforms this task into a straightforward reaction. The advantage: the reaction forms simple by-products—hydrogen chloride and sulfur dioxide. Both are gases, so they drift off during the process without muddying up what you want from the start.
In pharmaceuticals, these conversions can set the stage for pain relief medicines, antibiotics, and antiviral compounds. The reliability of SOCl2 matters each time a new medicine gets its start inside glassware. Many medicines that end up on pharmacy shelves owe something to the formula SOCl2.
In batteries, particularly lithium–thionyl chloride batteries, the compound finds a different calling. Here, it supplies high energy density and extended shelf life. GPS trackers, utility meters, medical devices—all benefit from batteries crafted with materials like SOCl2 inside. Those longer runtimes make a difference for business owners trying to avoid frequent and expensive maintenance trips.
Shortcomings and Safety Concerns
SOCl2 creates real risks. Most of us never want a cloud of its fumes drifting through the room, and for good reason—hydrogen chloride and sulfur dioxide irritate airways and can damage tissue. Accidental spills inside a lab or factory spark a scramble for clean-up. The formula’s strength means it shouldn’t land in untrained hands or poorly ventilated rooms. Even a small mistake can make a big mess and put people in danger of serious harm.
Improper storage also spells trouble. SOCl2 reacts with water—sometimes even from air moisture. Drums and bottles containing it need careful sealing. Splash-resistant gloves, safety goggles, and fume hoods make a day’s work safer. People who don’t take those steps risk chemical burns or breathing problems. As someone who once watched a careless coworker tip a bottle, I can tell you, splashing just a few drops generates clouds no one wants to deal with.
Possible Ways Forward
Safety training—regular and rigorous—needs attention in places where SOCl2 gets used. Lab managers who check in on safety standards build better habits for everyone. Industry groups share guidelines about storage, handling, and spill response. Buying high-quality protective equipment pays off in the long run. It’s also worth investing in modern ventilation. Smart designs for containers keep moisture away from the liquid, cutting down on the chance of surprise reactions.
Research into alternatives continues. Some labs experiment with other reagents for chlorination, weighing the risks and benefits. While SOCl2 holds its place for now, pressure grows for improvements and new options. Investing in these efforts may spark safer processes and greener chemistry down the road.
Why We Should Pay Attention
Understanding what SOCl2 can do—and what it can risk—gives everyone a better view of the intersection between necessity and responsibility. Even if you’ve never poured a chemical in your life, the ripple effects from chemistry labs reach everyday products and technologies. Keeping those processes safe and efficient helps us all, from scientists in the lab to the rest of us buying batteries at the store.
A Chemical Worth Extra Caution
Thionyl chloride shows up in many industrial and laboratory processes. As a chemist, I’ve handled this chemical with respect—and more than a little concern. Walk into any working facility, and you’ll hear the same advice: take thionyl chloride seriously. The reason is straightforward. This compound reacts violently with water and throws off toxic gases, even if just a little moisture sneaks in. For people working day in and out with chemicals, storing thionyl chloride correctly isn’t some bureaucratic box to tick. It’s a matter of safety, health, and keeping operations smooth.
Moisture: Thionyl Chloride’s Worst Enemy
Years back, an old drum in a storeroom corroded at the seam. No dramatic scene—just a slow, steady leak that made the air burn your nose. It took a few minutes to trace, but the lesson stuck with everyone on shift. Thionyl chloride meets water, even from room humidity, and it spits out sulfur dioxide and hydrogen chloride. Both gases are toxic. This makes storage practice more than just a technical detail. It directly protects people and the environment around them.
What Proper Storage Looks Like in Real Life
Only tightly sealed containers keep thionyl chloride safe from moisture. Most labs and factories use cylinders or drums made from steel lined with glass or special plastic coatings. Ordinary steel rusts out fast with this stuff. Polyethylene or Teflon liners stop corrosion, and glass seals provide the ultimate barrier for small quantities. I've seen colleagues get lazy with the lids on containers, only to spend an afternoon cleaning up a dangerous mess. Securing the cap isn’t optional.
Storing thionyl chloride away from heat helps, since higher temperatures drive up the pressure in containers and can increase the risk of leaks. Setting it in a cool, dry chemical storeroom with climate control makes all the difference. Putting it next to acids, bases, or chemicals that release lots of moisture spells trouble. Experienced techs check for leaks every week or after storms when air humidity can climb.
Proper labeling matters too. Simple, bold markings warn everyone of what’s inside every drum or flask. In a crowded storeroom, mistaken identity can cause disaster. Clear signage for dangerous chemicals keeps accidents from slipping under the radar.
The Human Factor: Training and Vigilance
No storage rule works without people who follow them. Regular training and reminders save lives. When someone new joins the lab, showing them the right storage routine for thionyl chloride quickly becomes part of their orientation. Over years, I’ve seen the difference this makes: from the more relaxed attitudes at smaller, less monitored shops, to the rigorous checklists at established facilities. The places with top safety records always have hands-on procedures, not just binders on a shelf.
Next Steps: Safer, Smarter Practices
Better storage means fewer spills, less emergency maintenance, and more confidence in the safety of the workplace. Investing in updated containers, dehumidifiers, and strict routines pays off every day. Senior chemists should never assume everyone knows the drill—mentoring new workers and calling out shortcuts keep everyone safer. Reporting near-misses, no matter how minor, sharpens collective awareness. The importance of careful thionyl chloride storage isn’t a theory—it’s visible in every safe, well-run workplace.
A Closer Look at Everyday Dangers
Thionyl chloride shows up in a lot of places—factories making pharmaceuticals, labs working on dyes, batteries, or even pesticides. It sounds like just another unremarkable chemical, but my work in chemical safety has made one point loud and clear: thionyl chloride doesn’t play around. Getting to know the hazards can make the difference between a safe workspace and a disaster with long-term consequences.
Breathing Trouble and Eye Burns
A splash or a spill will make you believe in goggles and good ventilation. Thionyl chloride fumes sting your eyes and throat right away. Even a little vapor can bring on coughing, chest tightness, and watery eyes. I’ve seen seasoned lab workers forced to leave the room, choking and coughing. Chemical safety agencies say breathing it in can burn the tissues inside your nose, trachea, and lungs. Exposing your skin or eyes does real damage—chemical burns that might need a hospital visit.
Contact with this liquid creates hydrochloric and sulfur dioxide gases, both corrosive and toxic. Hours after an accident, people can go from “just a little chest pain” to severe breathing trouble, sometimes leading to conditions like pulmonary edema, where fluid enters the lungs. That risk does not always show up right away.
Fire and Explosion
Factories keep thionyl chloride away from water for a reason. Any spill mixing with water means thick white clouds and a violent reaction—booted up by acids and heat. If a fire breaks out, things escalate fast. The fumes turn even nastier, making rescue or containment much harder. I’ve witnessed small leaks ignite panic and force full evacuations.
Environmental Mess
Spills don’t just stop at hurting people. When thionyl chloride leaks into soils or waterways, it eats through metal pipes, soil, and rocks—leaving behind acids and sulfur compounds that stick around in the environment. Wildlife, plants, and eventually people all get caught in the chain. Drinking water contamination brings big cleanup bills, lost production, and years of monitoring local wells. Real-world accidents prove that cleanup costs always outrun prevention expenses.
Lasting Health Effects
Pain from a spill heals, but damage to nerves, lungs, and eyes can linger for years. There’s no vaccine or quick fix for chronic exposure. Workers struggling with lifelong asthma or reduced lung function are not rare in places with poor controls. All it takes is missing one warning sign or skipping one safety step.
Better Safety Isn’t Optional
I’ve learned to respect thionyl chloride from seeing accidents that broke routines and lives. Wearing the right gear—goggles, gloves, and a protective suit—reduces direct contact. Fume hoods, scrubbers, and sealed systems cut down on vapor exposure. Training must be hands-on and repeated so everyone remembers what’s at stake. Simple steps like chemical storage in cool, dry places and using containers that don’t corrode can stop a big accident before it starts.
Every workplace needs clear rules and easy-to-find emergency showers, eyewash stations, and spill kits. No chemical labels hidden behind boxes, no shortcuts when mixing or disposing.
Hazard comes with the job, but trusted systems save lives. Hard-earned experience points to practical solutions: respect strong chemicals, never skip safety steps, and demand real training for everyone on site. The cost of ignoring thionyl chloride isn’t just measured in dollars; it’s people’s health and futures on the line.
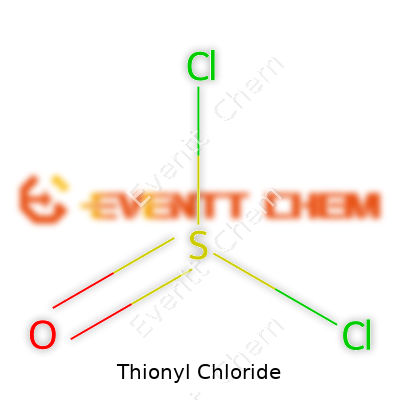

| Names | |
| Preferred IUPAC name | thionyl dichloride |
| Other names |
Sulfur oxychloride
Sulfurous oxychloride Sulfinyl chloride SOCl2 |
| Pronunciation | /ˈθaɪ.ə.nɪl ˈklɔːraɪd/ |
| Identifiers | |
| CAS Number | 7719-09-7 |
| 3D model (JSmol) | `JSmol` 3D model string for **Thionyl Chloride (SOCl2)**: ``` SOCl2 ``` *(This is the precise string to use as input in JSmol to show Thionyl Chloride.)* |
| Beilstein Reference | Beilstein Reference: 969677 |
| ChEBI | CHEBI:13281 |
| ChEMBL | CHEMBL1358 |
| ChemSpider | 11745 |
| DrugBank | DB15627 |
| ECHA InfoCard | 100.030.450 |
| EC Number | 231-748-8 |
| Gmelin Reference | 878 |
| KEGG | C07398 |
| MeSH | D013875 |
| PubChem CID | 1098 |
| RTECS number | WT5580000 |
| UNII | 47O27F7R2L |
| UN number | UN1836 |
| CompTox Dashboard (EPA) | EPA CompTox Dashboard: "DTXSID3023546 |
| Properties | |
| Chemical formula | SOCl2 |
| Molar mass | 118.97 g/mol |
| Appearance | Colorless to pale yellow liquid with a pungent, suffocating odor |
| Odor | Pungent, suffocating odor |
| Density | 1.638 g/cm³ |
| Solubility in water | Reacts violently |
| log P | -0.54 |
| Vapor pressure | 19.8 kPa (20 °C) |
| Acidity (pKa) | -7 |
| Basicity (pKb) | -7.05 |
| Magnetic susceptibility (χ) | -45.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.515 |
| Viscosity | 0.401 mPa·s (25 °C) |
| Dipole moment | 1.90 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –296 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -296 kJ/mol |
| Pharmacology | |
| ATC code | null |
| Hazards | |
| Main hazards | Toxic by inhalation, causes burns, reacts violently with water, releases toxic gases (SO₂, HCl). |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS02,GHS06,GHS05 |
| Signal word | Danger |
| Hazard statements | H314, H331, H302, H335 |
| Precautionary statements | P223, P261, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P335+P334, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-0-W |
| Autoignition temperature | 278 °C |
| Explosive limits | Not explosive as per GESTIS. |
| Lethal dose or concentration | LD₅₀ (oral, rat): 2600 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 141 mg/kg |
| NIOSH | WC4900000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Thionyl Chloride: "1 ppm (5 mg/m3) as ceiling |
| REL (Recommended) | 1 ppm (as a time-weighted average) |
| IDLH (Immediate danger) | 20 ppm |
| Related compounds | |
| Related compounds |
Sulfur dichloride
Disulfur dichloride Phosphoryl chloride Sulfuryl chloride |



