Sodium Acetate Trihydrate: More Than Just a Chemical Compound
Historical Development
Looking back, sodium acetate trihydrate’s story can start in the labs of Europe, where chemists first crystallized acetic acid salts in the 19th century. The understanding grew out of broader work into organic acids and fermentation, especially since vinegar production directly provided the feedstock. For decades, sodium acetate saw steady if unremarkable use, mainly tied to textile dyeing and food preservation. By the early 1900s, its refrigeration property got the spotlight—chemist’s notebooks from the era describe curious exothermic reactions and reuse as a heat pack. Manufacturing methods improved over time, from boiling wooden chips with caustic soda to modern chemical syntheses, showing how practical needs drive innovation.
Product Overview
Plenty of folks see sodium acetate trihydrate just as a white, granular solid dumped in factory drums. Those who have worked with it know its value stretches far deeper. Produced by combining acetic acid and sodium carbonate or sodium hydroxide, followed by proper crystallization, the result delivers reliable purity for both technical and food grades. Its ease of storage and long shelf life keep it on hand in research, medical, and industrial stocks worldwide. Its role in “instant heat packs” brings comfort to millions, not just chemists.
Physical & Chemical Properties
This solid appears colorless or white, draws water from the air, and melts around 58°C. It dissolves easily in water, forming a slightly basic solution. The trihydrate’s formula (C2H3NaO2·3H2O) shows three water molecules bound physically, which means it can lose them readily under heat—allowing supercooling and the famous “hot ice” trick science teachers demonstrate. The vinegar-like aroma signals its acetic heritage. With a density around 1.45 g/cm3, sodium acetate trihydrate can fill both bulk mineral shippers and precise laboratory vials.
Technical Specifications & Labeling
I’ve handled enough chemical sacks to respect a clear label. Here, reputable suppliers state purity, which can reach 99% for analytical needs or sit lower for deicing and bulk uses. Key specs—such as moisture, heavy metals, and residual acid—define its role in foods or labs. Labels must outline batch identification, storage advice (keep dry, sealed), and hazard notation. Global harmonized system (GHS) symbols often appear, ensuring workers spot risks even if language barriers exist.
Preparation Method
The route most chemists use involves reacting acetic acid (from vinegar or synthetic sources) with baking soda or sodium hydroxide. This mixture gets stirred, heated, and then cooled to form crystals. For higher-grade stock, manufacturers filter the solution, evaporate water slowly, and harvest crystals under controlled conditions. I’ve watched smaller labs make their sodium acetate by mixing household vinegar and baking soda in beakers, running the process much like those working in more industrial settings, only scaled down and without the automated drying tunnels and centrifuges.
Chemical Reactions & Modifications
Sodium acetate participates in plenty of useful reactions. It acts as a buffer in biochemistry labs, stabilizing pH levels. Reacted with strong acids, it releases acetic acid—useful for flavoring and preservation. In organic chemistry, heating sodium acetate with soda lime yields methane, a demonstration of decarboxylation. Less known but hugely important: Its ability to “salt out” DNA makes it a core tool for molecular biology labs everywhere. The compound’s trihydrate form lends versatility, since gentle heating shifts it towards the anhydrous solid, valuable in dry blends or pharmaceuticals.
Synonyms & Product Names
Depending on context, you’ll find sodium acetate trihydrate called sodium ethanoate trihydrate, vinegar salt, or even hot ice. Industries sometimes label variations by grade: ACS, food, or tech. It all boils down to the same molecular structure. Nomenclature can seem a tangle—trade brands pop up, yet the fundamentals remain easy for anyone with basic chemistry literacy to recognize.
Safety & Operational Standards
Decades spent in labs teach a deep respect for safe handling. Sodium acetate isn’t classified as a high-hazard compound—its main threats involve dust inhalation and mild irritation if it gets on broken skin or in the eyes. Proper gloves, safety glasses, and marked containers cut those risks. Facilities keep it dry and separated from acids. In the workplace, Material Safety Data Sheets (MSDS) provide quick reference, outlining what to do in case of spills or accidental exposure. Good ventilation and clear instructions make preventable accidents rare.
Application Area
The reach of sodium acetate trihydrate touches industries as varied as concrete construction, textile dyeing, food seasoning, heat storage, and biochemical research. Its most famous trick—crystallizing to store and later release heat—makes it a staple in reusable heating pads, especially in physical therapy or outdoor gear. Food processors use it for flavor (“salt and vinegar” snacks owe their punch to this material) and preservation. Ice control on airport runways often calls for sodium acetate, as it melts snow without corroding surfaces. Molecular biology and pharmaceuticals lean on it in DNA extraction and as a reaction buffer, where its stability trumps flashier chemicals every time.
Research & Development
Recent years show that sodium acetate trihydrate still sparks curiosity. Material scientists study its phase-change properties for greener heating and cooling methods. Biochemists refine buffer systems for genetic engineering using this salt. Concrete specialists improve road durability by adding sodium acetate, helping resist winter damage. Some researchers push into the realm of smart materials, using sodium acetate’s physical transformations for self-heating textiles or self-thawing coatings—combining chemistry tradition with new engineering vision.
Toxicity Research
For all its uses, sodium acetate trihydrate poses little acute risk. Animal studies back up its general safety, with toxicity levels much higher than typical workplace or consumer exposures. Ingesting large amounts can disrupt body pH, and long-term contact can cause skin dryness. Proper science means not ignoring minor hazards—even safe-seeming compounds deserve respectful use. Research tracks environmental impacts and finds quick breakdown in soil and water, helping keep its ecological footprint light.
Future Prospects
The next chapter of sodium acetate trihydrate could revolve around sustainability. Engineers examine its phase change for affordable thermal energy storage—which might cut residential heating bills and reduce fossil fuel dependency. Food technologists look to push the boundaries of “clean label” preservatives and flavorings, while pharmaceutical researchers focus on advancing delivery of therapeutics using its buffering abilities. The concrete industry might lean more on sodium acetate to fight infrastructure decay as urban populations grow and face harsher climates. My own experience convinces me that even an old standby can keep surprising us, especially when curiosity and real-world problems combine.
What You Actually Find It Doing
Sodium acetate trihydrate seems like one of those chemicals you forget about after high school. In real life, it shows up in places people rarely expect. I remember my first encounter with it came years ago in a classroom, watching a “hot ice” demonstration. A teacher clicked a metal disk and suddenly a liquid crystallized then warmed my palm. That small moment opened a door; it let me see why people keep reaching for sodium acetate trihydrate in laboratories, factories, and even kitchens.
Industry Relies on Simple Reactions
Some jobs ask for straightforward solutions to complex problems. Textile factories use sodium acetate trihydrate to neutralize sulfuric acid waste. When cellulose dyes are fixed onto fabric, acid seeps into the wastewater. Sodium acetate steps in, taming that acid so water treatment works right. The same compound helps chrome tanning in leather making, giving leather that reliable softness without corroding equipment. Industry isn't glitzy, but these jobs matter to millions who wear cotton shirts and leather shoes every day.
Everyday Science Hiding at Home
People love stories about kitchen chemistry. Sodium acetate isn’t a daily ingredient, but it can stop brined cucumbers or vegetables from getting too sour. Its mild saltiness and lack of intense flavor let it stabilize pickling solutions. Food scientists rely on it for more than taste, too—it acts as a shelf life extender by controlling pH and holding off bacteria better than table salt on its own. It earns its keep by stepping in quietly, making sure food stays safe longer. According to published research, sodium acetate’s antimicrobial and buffering qualities give food manufacturers an edge, especially for ready-to-eat items.
Medical and Science Tricks
Hospitals and first responders pick sodium acetate trihydrate for its instant heat. You can slap one of those reusable hand warmers on a wound or slide it into a hypothermia kit. Its liquid-crystal transformation isn't a gimmick—heat given off during solidification can save fingers or help relax sore muscles. In clinical labs, people add it to test tubes to precipitate DNA from solution, speeding up genetic research and medical testing. It looks like a simple compound on a label, but inside modern science, it saves time and improves accuracy. My own experience in a busy lab showed that a cheap, safe chemical with this many uses always finds a home in a toolbox.
Environmental and Waste Concerns
I’ve seen chemical suppliers tout sodium acetate as a “green” solution since it breaks down into safe byproducts compared to some older neutralizers. This isn’t perfect, though. Like most sodium-based salts, too much could add up in waterways and affect soil. Research from environmental agencies suggests small factories and labs should track disposal to avoid saline buildup, which threatens freshwater systems. Simple recycling or using it up completely for heating pads offers paths forward. We need more conversations between chemical suppliers, city planners, and engineers so one person’s fix doesn’t turn into someone else’s problem downstream.
Keeping It Useful and Safe
Sodium acetate trihydrate doesn’t chase headlines, yet its uses show up quietly every single day. I keep an emergency hot pack in my hiking kit, and after learning where it comes from, I see some products with new appreciation. What ought to drive all of us: match its convenience with practical disposal and thoughtful sourcing. The science and the stories behind this unassuming salt are worth knowing, if only because the simplest materials sometimes make the biggest difference where we least expect them.
Straight Talk on Safety
Sodium acetate trihydrate pops up in science labs, heat packs, and even in textile processes. It’s a salt that looks innocuous — often clear, sometimes powdery, often crystalline. The big question often circling around is: is it safe to handle?
Pulling from my own time sweating through chemistry classes and juggling chemicals in various settings, I can tell you that many people treat sodium acetate trihydrate as relatively safe compared to heavy hitters like strong acids or household bleach. Most laboratory manuals tag it as low risk, and you’ll frequently see bare hands scooping it into beakers or packing it into reusable hand warmers. That doesn’t mean reckless handling gets a green light.
Fact Check: Known Hazards and Sensible Practice
It can cause mild irritation, especially to skin and eyes. Play catch with the crystals and you’re likely to get away with it — but rub your eyes or skip handwashing, and irritation can kick in. Inhaling dust isn’t pleasant either. Lungs can feel scratchy, throats go dry. The U.S. National Library of Medicine and the European Chemicals Agency both back this up: no panic, just respect it as you would kitchen cleaning powder. Long-term health risks aren’t flagged, so you’re not looking at a hidden toxic time bomb.
Eating a bit won’t send you to the hospital, though nobody recommends it as a seasoning. The FDA actually lists sodium acetate as a safe food additive in tiny amounts, playing a role in flavoring potato chips and regulating acidity. That doesn’t translate to eating it by the spoonful on purpose. Too much isn’t healthy for kidneys and can mess with your electrolyte balance, as with most salts.
Solid Safety Habits Build Trust
Lab teachers always say: gloves, goggles, and a lab coat whenever chemicals come out. Overkill? Not really. Accidents show up when you least expect. One moment you’re cleaning up, next thing you know, some salt ends up in your contact lens. I remember seeing a friend get a stinging surprise this way, and wishing we’d actually listened to our safety instructor more closely. Whether you’re a student in a high school lab or an employee at a food production plant, treating all chemicals with thought goes a long way to earning the trust of your coworkers and keeping yourself healthy.
Disposal often gets ignored. It gets poured down the drain most of the time, since municipal systems handle this stuff just fine. Still, spree dumping buckets down the sink isn’t wise anywhere near drinking water sources. Always check local guidelines. Good practice means watching out not just for yourself but for those downstream, literally and figuratively.
What Actually Matters Most
Knowledge works as the best barrier against harm. Training programs, clear signage, up-to-date safety sheets, and some old-school supervision help keep things smooth. Nobody learns safe handling in isolation. Strong routines around chemicals, even apparently gentle ones, create a culture where safety’s the default, not an afterthought.
Sodium acetate trihydrate plays a role in education, research, and industry. The takeaway: it wants respect but doesn’t require fear. Build habits that treat every chemical with care. That way, curiosity turns into safe discoveries instead of unwanted mishaps.
The Formula at a Glance
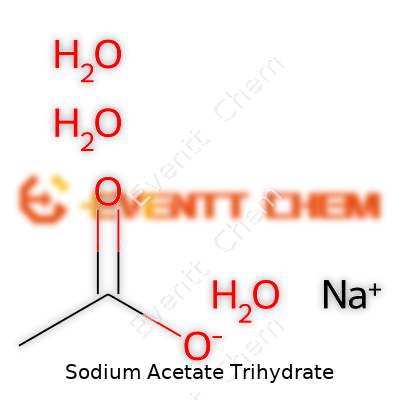
Sodium acetate trihydrate comes with a formula that reads CH3COONa·3H2O. In common terms, this means one sodium acetate molecule traps three water molecules in a neat structure. It's not just clever chemistry—a formula like this gives sodium acetate a range of uses, from laboratories to hot packs you can toss in your bag for a winter walk.
How this Chemical Shows Up in Life
I remember visiting a science workshop as a kid, where a volunteer snapped a metal disc inside a clear pouch and the liquid inside turned to a surprisngly hot solid. That was sodium acetate trihydrate doing its job. This salt, with its precise arrangement of sodium, acetate, and water, stores thermal energy. Click a starter, and you unleash that energy—like magic, but grounded in chemistry.
Beyond reusable hand warmers, industries use this compound to keep processes efficient and safe. Textile workers apply sodium acetate to set colors in fabric so clothing holds its hue even after piles of laundry. If you eat salt and vinegar chips, sodium acetate keeps the tangy flavor balanced. There's a difference between a theoretical compound and one found on shelves at grocery stores and hardware shops.
Why Formula Precision Matters
Getting formulas right isn’t only academic. Many disasters happen in industries and labs from mixing the wrong chemicals, even in small quantities. Sodium acetate trihydrate specifically means the chemical carries three water molecules in its crystal structure. Use anhydrous sodium acetate accidentally, and you won’t get that reliable release of heat in a hand warmer, or consistent chemical action in dyeing. Chemists and engineers trust these tiny details so equipment works, products perform, and safety holds up in the workplace.
Once, working on a kitchen science project, I tried substituting anhydrous sodium acetate for trihydrate, not realizing the difference. The reaction was off. The texture failed, and the project landed in the trash. Mistakes like that teach the value of formula accuracy more than any textbook page.
Building Trust: Purity and Quality
Mistakes and contamination can slip into products if suppliers cut corners or mislabel the product. Stories have emerged where companies order sodium acetate trihydrate, but impurities sneak in, resulting in unreliable performance. That's where responsible sourcing comes in—manufacturers with high standards reduce the risk of ruined batches or product recalls. It pays to check certificates of analysis instead of focusing on the lowest price.
Simple Solutions in Everyday Use
Clear labeling, supplier transparency, and hands-on education improve the safe use of sodium acetate trihydrate. Teachers can show students real-life differences between forms of compounds. Industry leaders support audits and share test results freely. Over time, this creates a culture where mistakes get caught before they reach the public. A little science solves practical problems—sometimes, chemical formulas make the world feel more understandable, one crystal at a time.
Why Storage Matters for Sodium Acetate Trihydrate
Anyone who works with chemicals learns the hard way—storage makes the difference between a material doing its job or turning troublesome. Sodium Acetate Trihydrate is no exception. It’s a solid that tends to pick up water from humid air, forming clumps or even dissolving into a gooey mess. Poor storage practices do more than waste product; they add unnecessary safety risks and expenses.
Key Environmental Factors to Watch
Moisture always creates problems for sodium acetate trihydrate. Exposure to open air in a damp room? The crystals attract moisture and cake together. I’ve seen bags start off free-flowing and end up with bricks inside when left on drafty shelves. So, a dry environment saves both time and money by protecting the chemical’s quality. Good air conditioning or dehumidifiers make a noticeable difference in storage rooms.
Temperature doesn’t cause much trouble for this material compared to moisture. It remains stable at ordinary room conditions. That said, hot warehouses speed up any caking if there’s humidity. Keep storage cool and dry, and contamination rarely happens. If I had to store it in an older building, I’d always keep the container sealed right after use.
Container Types and Best Practices
Plastic buckets with tight lids or thick polyethylene bags inside strong boxes give better protection than thin bags alone. I once saw someone use cheap, thin plastic liners. The chemical sweated right through after a week. Stick with solid plastic or metal drums with solid seals—costs more upfront, but saves on lost material later. Don’t forget the label, either. Clear labeling prevents mix-ups that lead to accidents or wasted raw materials.
Some labs keep sodium acetate trihydrate in glass jars for small-scale use, especially when precise weighing matters. Glass works fine as long as the cap seals completely. Avoid metal lids that rust over time in humid rooms. For my own bench, I found simple screw-top plastic jars keep the chemical dry enough for weeks.
Location and Safety Measures
Never place a sodium acetate trihydrate container near sinks or water sources. Splashes ruin the chemical. Shelves higher off the floor protect containers from accidental spills. If possible, dedicate a closed cabinet or storage closet for chemicals like this, away from direct sunlight and frequent traffic.
Spills happen, and sodium acetate trihydrate crystals become slick underfoot when wet. Always store it in easy-to-access spots, not balanced on high, wobbly shelves. Safe storage saves emergencies later. Food, drink, or any loose containers have no place near a chemical storage area.
Practical Solutions for Common Problems
Sometimes, storage rooms get damp for reasons no one can control. I’ve found that placing silica gel packets inside the bulk container soaks up stray moisture, especially during rainy seasons. Routine checks help too—open the lid, inspect for clumping, and reseal the jar. If the chemical has become damp, spread thin layers on trays and dry in a warm, dry oven before using it in sensitive processes.
It’s tempting to buy sodium acetate trihydrate in bulk to save money, but only do this if storage space stays dry year-round. Small, well-sealed containers get used up quickly and leave less room for error. Teach anyone handling the chemical to reseal containers right after taking their portion.
Why Good Chemical Storage Means Less Waste
Storing chemicals like sodium acetate trihydrate with care prevents wasted money and reduces headaches. Proper storage isn’t just about following a rulebook—it’s what keeps work running smoothly day after day. With good practice, those white crystals stay ready for whatever job comes next.
The Real Shelf Life: Why It Matters
Every lab tech and production manager I’ve worked with knows you can’t just stash chemicals away and expect them to keep forever. Sodium acetate trihydrate stands out as one of those compounds you find yourself returning to again and again, in classrooms, factories, and even cooking science demos. Its shelf life isn’t just a formality buried in a material safety data sheet—it’s a real concern for safety, cost, and reliability.
Sodium acetate trihydrate, with its delicate dance of salt and water, usually offers about three to five years of stability if stored right. Keeping it in a cool, dry spot with the lid on tight, protected from wide swings in temperature, helps a lot. Manufacturers generally print expiration dates on packaging not just to cover liability, but because this stuff draws in moisture and can break down faster if you let things slip. I’ve opened old bottles during inventory checks and found clumpy, sticky powder that didn’t look anything like what arrived years ago—not something you want in precision experiments or product manufacturing.
What Makes it Degrade?
Many chemicals start to misbehave if they get wet, warm, or exposed to air. That's especially true here. The “trihydrate” means each sodium acetate molecule hooks up with three water molecules, but this bond can break under poor storage, leading to evaporation or even conversion into a less useful compound. If you keep it in a humid warehouse or a lab where folks forget to reseal containers, the shelf life shrinks fast. Even day-to-day lab handling without care causes problems. Glass bottles I’ve used for years do a better job than plastic tubs that crack and leak.
Degraded sodium acetate trihydrate won’t catastrophically fail like some volatile chemicals, but its performance drops. This matters in food processing, chemical synthesis, and buffer solutions, where purity and performance directly affect the outcome. I’ve worked on student science projects that flopped simply because someone pulled an old, partly-dissolved bottle off a forgotten shelf. Frustrated students aren’t the main problem—mistakes in manufacturing or analysis could mean wasted batches or failed safety checks.
Testing and Longevity
Expiration dates should not replace good judgment or routine checks. Simple tell-tale signs—clumping, discoloration, an off smell—mean time to discard. Some labs run purity checks or use quick titration tests for assurance. I learned early on to label the date containers got opened, not just trust the printed expiration. This way, everyone knows exactly how long supplies have been exposed to the elements. Records prevent surprises, especially in regulated environments like pharmaceuticals or food labs.
Smarter Storage, Better Safety
We can get more life out of sodium acetate trihydrate through small changes. Buying smaller bottles cuts down waste. Using desiccant packs, good climate control, and airtight containers keeps material fresh. I’ve seen teams try to “rejuvenate” old material with drying agents—sometimes it works, but why risk it when fresher stock is affordable and reliable?
Ultimately, shelf life isn’t about squeezing every last gram from a container. It’s about protecting results, ensuring safety for users, and avoiding costly mistakes. Reliable storage and routine monitoring build peace of mind, for small labs and big operations alike.
| Names | |
| Preferred IUPAC name | sodium ethanoate trihydrate |
| Other names |
Acetic acid, sodium salt, trihydrate
Sodium ethanoate trihydrate Sodium acetate-3-water Ethanoic acid sodium salt trihydrate |
| Pronunciation | /ˈsəʊdiəm əˈsiːteɪt traɪhaɪˌdreɪt/ |
| Identifiers | |
| CAS Number | 6131-90-4 |
| 3D model (JSmol) | `3D structure (JSmol) string` for **Sodium Acetate Trihydrate**: ``` [Na+].CC(=O)[O-].O.O.O ``` This is the **SMILES string** representing the structure used in JSmol viewers. |
| Beilstein Reference | 3539536 |
| ChEBI | CHEBI:61307 |
| ChEMBL | CHEMBL1377 |
| ChemSpider | 5789 |
| DrugBank | DB09163 |
| ECHA InfoCard | 03b932e3-e9bb-4ac3-80a2-c286bfe1fe11 |
| EC Number | 204-823-8 |
| Gmelin Reference | 1567 |
| KEGG | C01738 |
| MeSH | D018432 |
| PubChem CID | 517044 |
| RTECS number | AJ4300010 |
| UNII | 57U80R16X6 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9036112 |
| Properties | |
| Chemical formula | NaC2H3O2·3H2O |
| Molar mass | 136.08 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.45 g/cm³ |
| Solubility in water | Very soluble |
| log P | -4.3 |
| Vapor pressure | <0.01 hPa (20°C) |
| Acidity (pKa) | 12.3 |
| Basicity (pKb) | pKb ≈ 9.25 |
| Magnetic susceptibility (χ) | -47.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.439 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.7 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 219.3 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1267.8 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1763.7 kJ/mol |
| Pharmacology | |
| ATC code | B05XA04 |
| Hazards | |
| Main hazards | Irritant to eyes, skin, and respiratory system. |
| GHS labelling | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 122 °C (252 °F; 395 K) |
| Lethal dose or concentration | LD50 Oral Rat 3530 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 11,900 mg/kg |
| NIOSH | SN1220000 |
| PEL (Permissible) | Not established. |
| REL (Recommended) | 60 mg/kg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Sodium acetate
Sodium acetate anhydrous Acetic acid Potassium acetate Calcium acetate |



