Potassium Acetate: A Close Look
History and Background
In the world of chemistry, potassium acetate isn't new. Early chemists recognized its value back in the 18th century, using it in laboratories long before industrial-scale production even appeared. By the 19th century, its benefits caught the eye of scientists working on salt mixtures for food preservation and textile dyeing. The transition from artisanal lab work to modern-day engineered compounds didn’t take long, and potassium acetate now fills a place in numerous fields. The journey from humble beginnings to a staple in laboratories and industrial sites builds a solid foundation and respect for what this compound can do.
What Sets Potassium Acetate Apart
Potassium acetate, known under names like E261 or dieter’s salt, steps in as a versatile salt formed by the neutralization of acetic acid with potassium carbonate or potassium hydroxide. Its formula, CH3COOK, may look simple, but it holds practical power well beyond that of sound-alike table salts. As a deliquescent white powder, it absorbs water right from the air. This hygroscopic nature keeps it flexible for experimental setups and de-icing operations. Unlike sodium salts, potassium acetate offers a kinder pathway for applications where sodium-sensitive materials or health concerns exist. Not only does it dissolve quickly in water, but it also provides a more environmentally friendly choice compared to chloride-based deicers.
Physical and Chemical Properties
Potassium acetate brings a subtle vinegar scent, owed to its acetic acid roots. The compound’s melting point sticks around 292°C before it breaks down, making it reliable in high-temperature settings. Water loves it; a solid dose will vanish in just a few milliliters. Ethanol joins the party too, though to a lesser extent. The salt comes with a pH between 7.5 and 9.0 for solutions, so it leans a bit to the basic side. This character opens the door to several chemical reactions that demand buffering capabilities but do not invite wild swings in acidity or alkalinity. Rich solubility and chemical stability protect performance across storage, shipping, and usage.
Technical Specifications and Labeling
Manufacturers work with strict controls to deliver potassium acetate at purity levels above 99%, depending on the demand. Food-grade types receive E-number labeling, showing a nod to legislation on additives and safety. Reagent grades target laboratories needing clean, predictable results. Bulk shipments list batch numbers, manufacturing dates, and hazard information, all aligned with international regulations. Packaging often features tamper-proof seals, plus clear dosage and storage instructions tailored to the product’s specific grade. This detail matters: errors in labeling can turn a safe compound into a major risk, especially in food, pharmaceuticals, or water treatment plants.
Preparation and Synthesis
In most industrial setups, producing potassium acetate means mixing glacial acetic acid with potassium carbonate or potassium hydroxide. The reaction gets lively, fizzing as carbon dioxide escapes. After filtration and controlled evaporation, crystals settle to the bottom and get scooped up for drying. This straightforward process offers no tricky intermediates, so manufacturers scale up production easily and cost-effectively. For those seeking the purest product, companies often add a recrystallization step, purging trace contaminants and ensuring top-grade salt in the end. Smaller labs may brew up batches on demand, valuing freshness and freedom from caking agents or bulk storage issues.
Chemical Behavior and Modifications
Potassium acetate’s straightforward structure brings consistent results in many reactions. A classic use crops up in organic synthesis, where it swaps potassium for other cations or drives the formation of esters in the presence of acid chlorides. Pairing it with strong acids or oxidizers demands respect—potassium acetate doesn’t take kindly to careless handling in those circumstances. Scientists also turn to modified forms, such as deuterated potassium acetate, in advanced nuclear magnetic resonance experiments, chasing down precise molecular information. Its readiness to participate in straightforward acid-base interactions keeps the compound flexible in daily tasks, from neutralizations to buffer creation.
Alternative Names and Labeling Practices
This compound hides behind a few other names: potassium ethanoate, AcOK, or E261 in food. Each sector lands on a preferred naming system. In chemical catalogs, potassium acetate appears with its Chemical Abstracts Service (CAS) number, and some product names carry batch codes or manufacturer references. The food industry leans on “E261” and lists its use as a preservative or acidity regulator, often substituting potassium acetate for sodium-based cousins to reduce health concerns tied to sodium intake. The same simple payout—potassium instead of sodium—lets buyers make informed, healthier choices, especially in processed foods or specialty diets.
Safe Usage and Workplace Practices
Potassium acetate doesn’t bring the same hazards as strong acids or heavy metals, but good practice stays important. Dust can irritate eyes or skin in bulk handling. Industrial sites often provide gloves, goggles, and local exhaust systems. Material safety data sheets break down emergency steps: rinse with water after contact, keep containers sealed, and avoid breathing in airborne particles. Food and pharmaceutical processors follow even higher standards, regularly checking for trace impurities, microbial activity, and anything that could compromise end-user health. Disposal—another angle that shouldn’t get ignored—funnels spent material to wastewater facilities that monitor for excess potassium, safeguarding both people and the environment.
Application Landscape
A walk through industrial sites or research universities shows potassium acetate playing vital roles in more places than most people imagine. De-icing claims a big chunk of demand, as this salt melts ice on runways and roads with less risk of corroding metals or poisoning aquatic systems than standard options like sodium chloride. Controls in fire suppression systems use potassium acetate-based extinguishers to safely knock down grease fires in kitchens and machinery bays. Laboratories rely on its gentle buffering action for molecular biology and biochemistry, where the focus rests on maintaining stable pH during DNA purification. Food processors use it as a preservative, often touting “lower sodium” right on labels of cheese, cured meats, and snacks. The pharmaceutical industry benefits too, sometimes using potassium acetate as a base-generating agent in effervescent formulations or as an alternative electrolyte to reduce sodium load in specialized treatments.
Current Research and Innovation
Research laboratories continue to look for better uses of potassium acetate. Energy storage studies explore whether potassium acetate’s unique properties can enhance cost-effective batteries. Some researchers examine agricultural applications, seeking ways to supply potassium to crops that need a sodium-free boost. Interest also grows in formulating new de-icing agents that work over broader temperature ranges, without harming the soil or groundwater. Molecular biology teams keep refining DNA extraction protocols using potassium acetate to boost purity, especially in tough environments where contaminants can cloud results. Pharmaceutical advances focus on gentler delivery systems for patients who need potassium but must avoid sodium. Each research step brings this compound closer to the next generation of applied science.
Studies in Toxicity and Human Health
Potassium acetate’s safety track record stands firm, but nothing goes unchecked. Research examines toxicity at multiple levels. Animal studies show that large doses can trigger gastrointestinal upset or disrupt normal potassium balance, but typical levels in food and consumer products stay far below that threshold. Human clinical trials and dietary surveys confirm its status as a safe sodium substitute on approved ingredient lists. Regulatory agencies set upper limits to defend against potassium overload, especially in groups with kidney problems or those already on potassium-sparing medications. Workplace exposure reviews and environmental monitoring make sure no stone is left unturned, balancing trust in established safety with a steady eye on emerging risks.
Looking Ahead: Future Potential
Potassium acetate presents a compelling future for sustainability and public safety. Cities grapple with ice and snow control, and potassium acetate offers pathways with fewer scars on infrastructure. Dieticians recommend the shift from sodium to potassium-based additives, nudging public health in a better direction. Research on incorporating potassium acetate in lithium-ion battery systems continues, with hopes that it can lower costs and environmental impacts. Some efforts even target biomedical applications, like wound care or intravenous fluid design, aiming for better potassium balance without unintended consequences. Teams focusing on green chemistry point toward potassium acetate as a possible building block in biodegradable packaging or biosafe solvents. Progress across these fields doesn’t come from hype, but from the real-world track record this compound quietly builds, making it an anchor for thoughtful innovation across science, industry, and medicine.
Cold Streets and Safer Skies: Clearing Up the Uses
Most folks walk over potassium acetate in the winter without ever noticing. City crews use it to melt ice on airport runways and busy roads. The usual product for battling ice, sodium chloride (table salt), can tear up pavement, hurt plants, and leave vehicles with rust spots. Potassium acetate fixes some of those headaches. It prevents concrete from corroding and doesn't leave the white sludge people track into lobbies. In places like Chicago O’Hare or Minneapolis-St. Paul, crews spray potassium acetate in frigid weather to help keep flights on schedule and runways safe. I’ve watched airport tarmac teams move in with big trucks filled with the liquid. Planes keep moving, and passengers avoid long delays.
Food and Lab Life: Where Potassium Acetate Shows Up Unexpectedly
Potassium acetate also heads into the kitchen—sometimes hidden in powdered mixes, processed cheeses, and pickled foods. Under the label E261, it acts as a preservative. No one lines up for a food additive parade, but it does its job without fuss, helping foods fight bacteria and spoilage. It brings one of the essential minerals, potassium, to the table. Most people get enough from bananas and potatoes, but a little extra slipped in from additives usually isn’t a big concern. The U.S. Food and Drug Administration considers this substance safe when used within limits. Too much can tip the balance for people with kidney problems, so labeling and smart formulation help guide producers and consumers alike.
A Helping Hand in Hospitals
Doctors and nurses sometimes use potassium acetate in intravenous fluids for patients who can’t take potassium pills by mouth. Potassium is crucial for muscles, including the heart, to keep working right. Too little causes weakness and abnormal heart rhythms. Some folks in critical care need tailored fluid mixes that treat them without causing excess acid or sodium buildup. Hospitals rely on precisely made solutions. Nobody wants to see an error with electrolytes—which might explain why clinical staff always check dosing and double-check labels before running a drip.
Sewers, Science, and Making a Difference
Cities also dose wastewater plants with potassium acetate. In sewage treatment, it helps friendly bacteria break down the waste quickly, especially during tough times like winter or when the waste mix changes. In the past, I worked on a project where a treatment facility fought to keep the process running right after a food processor dumped extra load. Adding potassium acetate pulled the system back into balance, kept odors down, and helped the city stay on the good side of environmental rules.
Fixing Overuse and Looking Ahead
There’s always a catch. Potassium acetate, if overused, can leach into groundwater. It costs more than rock salt, so city managers and maintenance teams have to balance budgets against environmental safety. Some airports have adopted sensors that spray just enough and shut off to limit run-off. I’ve seen training sessions emphasizing careful mixing and targeted spraying to keep operations tight and safe.
Potassium acetate rarely makes front-page news. Still, it shapes the world, from keeping planes on time to protecting city pipes, and does so quietly. Smart use and transparency about ingredients and environmental impact build trust between industries and the public. Solutions start with understanding what this unassuming salt can do—and where limits matter most.
Daily Use Versus Industrial Exposure
Potassium acetate shows up in a surprising number of everyday products, from road de-icing salt to food preservatives. In food, it often plays a behind-the-scenes role as an acidity regulator. I’ve seen it listed in snack labels and as a replacement for sodium-based salts on icy sidewalks. Most people pass by it without a second thought, especially since regulatory institutions like the FDA find it safe in food at low concentrations.
But let’s talk about real handling—big bags in an industrial setting, open containers in a lab, regular exposure by workers. This is where an honest look at safety matters most. Potassium acetate in its solid form and its solutions don't carry the same high risk as some other salts, like chromates or cyanides. It won’t corrode skin instantly or fume up a closed room. On the other hand, ignoring basic hygiene and safety leads to problems down the road.
Eyes, Skin, and Lungs: Sensible Protection
Direct contact with solid potassium acetate or a concentrated solution can irritate skin and eyes. I remember a colleague getting a splash to the hand, and after a thorough rinse, there was only some redness. Let it sit, though, and chafing or more persistent irritation can follow. Eyes handle it less easily—splash protection matters. Inhaling fine dust from bulk handling can cause coughing or throat irritation, so decent ventilation and dust masks make sense. Gloves, goggles, and lab coats or old work clothes rule out the chance of a moment's accident turning your whole day sour.
Fire Hazards and Chemistry Lessons
Potassium acetate stands out from sodium chloride: while not flammable itself, it can add fuel to a fire. In certain conditions, especially if the product is heated to decomposition, potassium acetate gives off smoke that contains acetic acid fumes. If anyone’s been near a vinegar factory’s exhaust, you’ll know sharp acetic smells leave a mark in your memory. Ventilated handling areas and smart storage keep this from turning into a major headache.
Accidents and Real-World Prevention
Serious harm doesn’t show up in safety data reports unless there's consistent neglect. Most cases involve momentary slips: ungloved hands, splashes, or dust clouds from cutting open bags. I heard about a small fire in a supply closet years ago where potassium acetate, present in containers, didn’t worsen the blaze directly but clean-up took extra steps because of the chemical runoff. Anyone running storage or handling areas should keep spill kits, running water, and fire extinguishers ready. Training workers not just with paperwork, but with hands-on walkthroughs, helps these procedures stick.
Supporting Safety With Knowledge
Labeling, hazard communication, and ongoing reviews stand as a workplace’s backbone. Those regular safety meetings may sound routine, but a clear emergency plan, clear labeling, and up-to-date safety data sheets keep workplaces protected against small mistakes turning bigger. A walk through OSHA guidelines for chemical storage spells this out clearly: knowledge, maintained habits, and shared vigilance lower risks for everyone.
Potassium acetate is pretty safe with common-sense steps: protect your eyes, cover your skin, keep out clouds of dust. Respect it like any chemical, keep an emergency plan close at hand, and handling it won’t cause trouble.
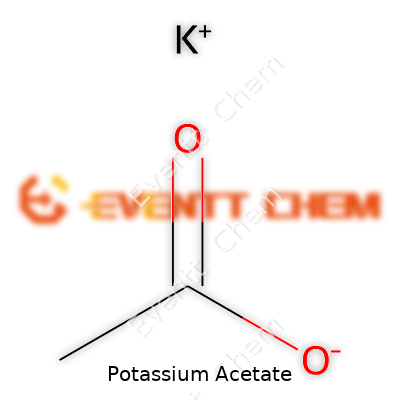
Potassium Acetate and Its Formula
Potassium acetate steps onto the chemistry stage with a straightforward formula: CH3COOK. In everyday life, hardly anyone goes around talking about it at the dinner table, but this simple mix of elements crops up in all sorts of surprising places: medicine, food processing, even in the stuff spread over icy roads.
What Makes This Formula Important?
Memorizing formulas in high school chemistry often felt like busywork, but understanding what CH3COOK represents packs a lot of practical value. Breaking it down, you get a potassium ion (K+) paired with an acetate ion (CH3COO-). This mix neutralizes acids and does a fine job helping maintain pH in systems that need stability, like blood in the body or solutions in a lab.
Take it from someone who’s slipped on a patch of black ice in winter—potassium acetate ends up saving more bones than you might think. City workers use it in de-icing blends because it’s gentler on concrete and the environment than some chloride salts. The formula also matters for food quality. Manufacturers add small doses to control acidity and extend the shelf life of everything from canned vegetables to processed cheese. That reliance on a safe, effective additive highlights the trust that industries place in accurate knowledge of chemical makeup.
Balancing Benefits and Risks
While potassium acetate seems pretty tame on paper, real-world use demands respect for both benefit and risk. Any time a chemical makes it into food, water, or the bloodstream, oversight keeps everyone safe. For example, doctors administer potassium acetate intravenously to treat low potassium in patients, but dosing needs precision. Too much in the wrong context, and you can tip the body’s electrolyte balance out of whack.
For the food system, regulators like the FDA and the European Food Safety Authority set strict guidelines. Research shows potassium acetate doesn’t build up excessively in the body, unlike other additives, but oversight keeps the risk in check. This approach helps avoid chronic exposure problems down the road.
Clear Communication Builds Trust
Talking chemistry with folks outside the lab often feels like wading through a swamp of jargon. You see long names and formulas, and it’s easy to feel out of the loop. But taking a chemical like potassium acetate and breaking it into simple terms—potassium plus acetate—helps everyone understand why it gets used in the first place. That kind of transparency fosters public trust, whether it’s on a food label or a bag of runway de-icer.
Experience shows that well-informed people make better choices. From a hands-on perspective, the value of openly stating what goes into our bodies and our communities can’t be overstated. More open labeling, regular testing, and clear answers from the experts keep missteps out of the headlines and confidence high at the grocery store or pharmacy.
Paths to Safer Use and More Knowledge
Regular updates to safety guidelines, and investment in public science education, form the foundation for practical chemical knowledge. If everyone from schoolkids to city planners learns how formulas link to function—what CH3COOK truly means—misuse shrinks, and safe, smart use grows. Industry and the public both get more from these chemical tools by sharing clear, accurate information.
Whether it’s melting ice, regulating a heartbeat, or keeping food tasting fresh, potassium acetate’s formula stands for more than just chemistry. It represents a shared stake in understanding what we use, and why it matters.
Why Potassium Acetate Calls for Attention
Most folks won’t find potassium acetate on a weekly shopping list, but this salt plays a role everywhere from airport runways to biology labs. It’s used to keep ice off concrete, to help make food safe, and to stabilize certain medicines. For people in the business of handling potassium acetate, knowing where and how it sits can make a big difference in both safety and product quality.
Safety Hazards Aren’t Just for Chemists
Under the wrong conditions, potassium acetate can clump up, draw in water from the air, or even become contaminated. Storing it in a clean, dry environment is not about being overcautious — nobody likes dealing with ruined supplies or cleaning up sticky messes from a leaking barrel. OSHA’s own documentation flags this compound as irritating to skin and eyes. Unsafe storage means emergency room visits and lost time, not to mention cash down the drain.
Container Choices Matter
From what I’ve seen, companies use everything from 25 kg bags to steel drums for bulk storage. I remember when a supplier used flimsy packaging, and the humidity in the storeroom quickly turned half the shipment into hard lumps. Air-tight containers, kept off concrete floors, block this problem. Rust never sleeps — so watch for corrosion if using metal containers. Labels should include clear hazards and dates to help avoid dangerous mix-ups.
Location, Location, Location
Potassium acetate shouldn’t sit just anywhere. Stacking it next to acids spells trouble, because if those containers get knocked over, you could get a violent chemical reaction. A designated storage area, with little chance for rough handling or collisions, cuts that risk. I’ve visited facilities that kept their chemicals near high-traffic paths, and accidents followed. Best practice means separate storage bays, organized by compatibility. Ventilation is another part that’s often skipped, but it helps clear up fumes and keeps everyone breathing easy.
No Place for Moisture or Heat
Too much moisture spells disaster. Potassium acetate draws in water from the air, becomes sticky, and sometimes leaks right out of the bottom of containers. Store it in rooms with low humidity and stable, room-level temperatures. Avoid spots near boilers, radiators, or windows with direct sun.
Training for Real-World Scenarios
Safety stories pile up in the news about folks mixing chemicals without the right prep. Everyone handling chemicals needs basic training about what potassium acetate can and can’t do. Things like using gloves, goggles, and knowing where emergency washes are located prevent small mistakes from becoming big news stories.
Record-Keeping Makes Life Easier
Logbooks might seem old-fashioned, but they keep everyone honest. Tracking when potassium acetate arrives, how it’s used, and when it’s running low keeps expired or degraded stock off the floor. Digital inventory systems flag problems faster, but a paper backup helps if the network goes down.
Better Storage Starts with Common Sense
Treat potassium acetate the way most people treat food: keep it sealed, cool, and away from stuff that could spoil it. Look into your storage areas now and then, checking for leaks, strange odors, or missing labels. Those simple steps can add up to a safer workplace — and keep important supplies in top shape.
Thinking About Solubility in Everyday Life
Let’s talk potassium acetate. This isn’t just a name out of a textbook or an ingredient buried in a bag of fertilizer. Every time you see a de-icer melt snow on winter sidewalks, there’s a good chance potassium acetate plays a part somewhere. With all this use, the question of solubility isn’t some academic quirk—it’s about the stuff we live with every winter and even in hospital settings.
What Happens in Water?
Pour potassium acetate into water and it dissolves easily. No need for vigorous stirring or special techniques. Kool-Aid dissolves in water because the sugar molecules can break apart and scatter among the water molecules; potassium acetate works on a similar principle at the atomic level. Lab resources and science handbooks show it mixes readily with water in both cold and warm conditions. This isn’t magic; potassium ions and acetate ions just want to spread out in water, leaving the solution clear with no lumps or crust.
Why Should Anyone Care?
If you’ve dealt with ice on runways or sidewalks, you might have seen potassium acetate at work. Cities and airports value its quick action and compatibility with concrete, which sets it apart from harsher road salts like calcium chloride or sodium chloride. Those options can erode concrete and damage steel over time. Potassium acetate’s high solubility makes it easier to apply, requiring less mechanical effort to distribute. Pilots benefit from knowing the runways are safe, while municipal workers avoid back-breaking labor.
Hospitals and clinics get another big benefit. Potassium acetate dissolves in saline or other IV fluids so it’s used to adjust electrolyte balances, especially in patients with low potassium. Safe, reliable mixing keeps medications and fluids accurate, which makes a real-world difference to patient care. Clinical references point out that it produces a clear solution every time, cutting error risks for medical teams. Efficiency here means less room for mistakes and more time for hands-on care.
Downsides and Environmental Concerns
Pretty much any substance used in bulk, especially for deicing, brings environmental concerns. Potassium acetate, dissolved in water, can eventually flush into the soil or waterways. Runoff can change how plants grow, sometimes throwing off the potassium balance in soil. High potassium loads may even harm aquatic living things if used without restraint. In practice, municipal workers and facility managers face tough choices balancing efficiency, cost and safety.
Solutions and Responsible Choices
Regular testing helps spot trouble before it magnifies. Soil and water checks limit the risk of accidental over-application, keeping those landscapes healthy. Application guidelines can be updated more often if city scientists and field operators share real results, not just theoretical advice. Educating snow crews, facility techs and even gardeners about smart usage keeps our urban systems running without unwanted side-effects. Hospitals, for their part, follow strict protocols so medical fluids don’t become a hazard to staff or patients.
Why the Chemistry Lesson Matters
Clear answers about solubility turn up in real moments on airport tarmacs, street corners and emergency rooms. Potassium acetate dissolves fast, spreads easily and stays reliable across a huge range of uses. Digging a little deeper into the science, and looking at all sides of the issue—including runoff and drug safety—means better decisions get made. In the end, asking about solubility doesn’t just fill a curiosity gap; it connects to real safety, public health and the world just outside your door.

| Names | |
| Preferred IUPAC name | Potassium ethanoate |
| Other names |
Acetic acid potassium salt
Potassium ethanoate E261 |
| Pronunciation | /poʊˈtæsiəm ˈæsɪteɪt/ |
| Identifiers | |
| CAS Number | 127-08-2 |
| Beilstein Reference | 635104 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1201767 |
| ChemSpider | 5663 |
| DrugBank | DB01310 |
| ECHA InfoCard | 01b112af-4606-49b1-b824-dc0e3f4f0235 |
| EC Number | 204-822-2 |
| Gmelin Reference | 6764 |
| KEGG | C02720 |
| MeSH | D019361 |
| PubChem CID | 517044 |
| RTECS number | AJ4300010 |
| UNII | CPD4NFA903 |
| UN number | UN 2570 |
| CompTox Dashboard (EPA) | urn:uuid:ee8c26ab-16c2-4cb8-8883-9e45fa6fe50f |
| Properties | |
| Chemical formula | KC2H3O2 |
| Molar mass | 98.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.57 g/cm³ |
| Solubility in water | 2535 g/L (20 °C) |
| log P | -2.55 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | 9.25 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.370 |
| Viscosity | 1.49 cP (20 °C, water solution) |
| Dipole moment | 1.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 86.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -576.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -925.8 kJ/mol |
| Pharmacology | |
| ATC code | B05XA15 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | > 593 °C (1099 °F; 866 K) |
| Lethal dose or concentration | LD50 oral rat 3250 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat): 3250 mg/kg |
| NIOSH | BS8450000 |
| PEL (Permissible) | not established |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
Sodium acetate
Calcium acetate Acetic acid Potassium carbonate Potassium chloride |



