Nickelous Acetate: A Deep Dive into Its Chemical Journey
Historical Development
Nickelous acetate didn’t show up out of nowhere. Its story traces back to the era when nickel extraction started scaling up in the nineteenth century. Early chemists stumbled on nickel compounds as they worked to separate metals from their ores. Getting nickel into a soluble form like nickelous acetate opened new doors in plating and dyeing, both of which found their stride during the industrial revolution. With time, researchers discovered nickel’s ability to anchor dyes on textiles and plate a durable layer on metal surfaces, transforming how everyday products got their shine or color. My own brush with it came in a college lab—nickelous acetate always helped students get a grip on electrochemistry, demonstrating the behavior of transition metal salts with vivid green solutions.
Product Overview
Nickelous acetate holds its place as a staple in both academic and industrial labs. Recognized for its green crystals, it acts as a backbone for many nickel-based syntheses. In bottles lining classroom shelves and production lines, this salt turns up as both a teaching tool and a building block. Technician and researcher alike reach for it when the goal is cleanliness and predictable results, especially in plating, catalyst prep, and analytical chemistry. Its combination of reliability, availability and manageable pricing keeps it in regular rotation in so many projects.
Physical & Chemical Properties
This compound appears as pale green crystals that dissolve easily in water and alcohol, putting its ions to work without much fuss. The chemical formula Ni(CH3COO)2·4H2O describes the common hydrated form, which comes loaded with four water molecules for each nickel atom. Chemically, the acetate ions loosen up as the mineral hits water, releasing nickel(II) cations that give the solution its color and its hefty reactivity. The crystals feel slightly sticky, they absorb moisture from the air, and break down at high temperature to form nickel oxide, setting off nasty acetic acid fumes in the process. These traits give a hint at why proper storage and handling make such a difference.
Technical Specifications & Labeling
Industry standards guide the trade and use of nickelous acetate: a guarantee of purity (usually over 98% for laboratory grade), a precise nickel content not far off 22%, and controlled levels of other metals like iron or copper that can skew sensitive processes. Packaging sports warning labels about toxicity and environmental impact, reminding users of the need for gloves, goggles, and good ventilation. Many labs take these reminders to heart, running spot checks on batches to keep their experiments and final products up to scratch.
Preparation Method
To make nickelous acetate, manufacturers start by dissolving either nickel carbonate or nickel hydroxide in acetic acid. Anyone who’s spent a day in a wet chemical lab knows the distinct smell that wafts up once vinegar meets metal. The process gets fined-tuned: control the acid’s strength, filter out any undissolved junk, evaporate the water to concentrate the mix, and let crystals form on cooling. These steps don’t just happen in big refineries; college labs sometimes run them as demonstration experiments, helping new chemists gain respect for careful handling and purity.
Chemical Reactions & Modifications
Nickelous acetate does more than sit idle. It acts as a starting block in multiple reactions. Combine it with sodium borohydride or hydrazine, and you’ll get metallic nickel—a key step in producing catalysts and advanced materials. In cross-coupling chemistry, especially Suzuki or Heck reactions, nickelous acetate works as a pre-catalyst, helping organic chemists stitch together tricky carbon frameworks. Acetate ions can be swapped out for others to get nickel chloride or nickel nitrate simply by trading acids, giving chemists a flexible toolkit. This adaptability keeps labs stocked with nickelous acetate on standby for quick adjustments.
Synonyms & Product Names
Anyone who shops for chemicals runs into a variety of names. Nickelous acetate also gets listed as nickel(II) acetate, nickel diacetate, or acetic acid nickel(II) salt. Catalogs sometimes add extra detail—tetrahydrate, just to clarify the form. These alternate names can cause headaches if you’re hunting for a specific grade or water content, but once you’re hands-on with the materials, the differences become clear from the labeling and technical sheets.
Safety & Operational Standards
Working with nickelous acetate doesn’t leave much room for shortcuts. The salt creates dusts and solutions that can irritate skin and eyes or set off sensitivities after repeated exposure. Some countries mandate a fume hood for any open processing. Disposal values human and environmental health just as highly—nickel compounds shouldn’t find their way into regular drains, so used solutions require collection and treatment at specialized facilities. In my experience, workers respect this material. Safety data sheets remind teams to double bag solid waste, wash up thoroughly, and keep emergency eyewash stations stocked. There’s a sober recognition in every lab—nickelous acetate’s usefulness rides on the back of strict safety discipline.
Application Area
Nickelous acetate plays a role in diverse fields. In electroplating tanks, it helps deposit uniform layers of nickel onto metals, preventing rust and lending a bright, appealing finish to everything from coins to tools. Textile factories use its predictable chemistry to fix colors firmly during dyeing. Laboratories use it to prepare other nickel compounds for further experiments in catalysis or material science. In battery development, research teams use nickelous acetate to craft specialized electrodes and test new energy storage concepts. It gives engineers a way to boost material performance in industries that stretch from electronics to aerospace.
Research & Development
Research into nickelous acetate keeps evolving. Teams study its complexation behaviors and optimize reaction pathways, hoping to eek out higher yields, better purity, or new properties. Some researchers focus on how it interacts with organic ligands, opening up chances for custom catalysts. Others look into green chemistry—can we cut energy use, switch to renewable acetic acid sources, or reduce waste by refining crystal growth processes? In my postgraduate days, watching teams modify nickelous acetate for more efficient chemical vapor deposition drove home just how much even familiar compounds can surprise when tools and ideas shift with technology.
Toxicity Research
Nickelous acetate doesn’t just offer utility—it demands caution. Toxicological tests show it can cause respiratory irritation, skin rashes, and, after long exposure, contribute to cancer risk. Inhalation and ingestion pose threats for both lab workers and the public if spills or improper handling occur. These facts underscore why regulations grow tighter every year. Laboratory studies into its environmental fate show nickel ions linger in soil and water, disrupting ecosystems if unchecked. As a result, health and safety experts urge regular monitoring, use of closed systems, and continuous reevaluation of exposure limits. There’s a clear push to balance usefulness with responsibility.
Future Prospects
Looking ahead, nickelous acetate seems poised for even broader relevance. Its role in batteries—especially for electric vehicles and renewable energy storage—drives much of the excitement in both industry and academia. Scientists work to refine its synthesis for greener outputs, hoping to create more sustainable nickel resources. Teams push for more efficient recycling of nickel-containing waste and safer alternatives for the most hazardous steps in its life cycle. New uses in catalysis, especially for cutting-edge pharmaceuticals or eco-friendly polymers, catch interest as new reaction pathways become possible. As society focuses more sharply on green technology and safe, scalable manufacturing, nickelous acetate stands ready for reinvention yet again.
Out in the Real World
Nickelous acetate might seem like just another chemical with an obscure name. In real life, this compound plays a part in many things most people handle every day, often without ever realizing it. I remember seeing piles of coins and thinking, “Who decides how these stay shiny for years?” For anyone who has strolled through a hardware store and eyed rows of steel tools, or has old coins saved in a jar, nickelous acetate has already left its mark.
Helping Metal Stand Up to Wear
Most folks don’t realize how quickly steel and copper break down when moisture and oxygen hang around. Nickelous acetate helps solve this problem through a method called electroplating. In electroplating, a thin layer of nickel covers other metals. Nickelous acetate works as the source of nickel during this process. The nickel surface keeps away rust and brings a bright tone to bathroom fixtures, kitchen utensils, and even bike parts.
Big manufacturers lean on this chemical because it also increases how much stress metals handle before bending or breaking. A nickel coating from a solution with nickelous acetate lasts longer under friction, rain, or even the heat of an engine. This isn’t only about looks; I’ve seen older tools that lasted for decades, thanks to nickel plating. Without it, replacing corroded gear would pile on costs and create more waste.
Coloring Glass and Ceramics
Artisans turn to this nickel salt for coloring glass and ceramics. Mixing the compound into molten glass brings soft green and blue shades that pop in sunlight. Ceramic tiles, pottery, and decorative glazes benefit, too, helping them stand out on shelves or in kitchens. This practice mixes industrial science with artistry.
Inside Batteries and Chemistry Labs
Nickelous acetate supports several types of batteries, including rechargeable nickel-cadmium and nickel-metal hydride batteries. Both types store power for gadgets that run every household—from cordless tools to flashlights. Chemists often grab nickelous acetate in labs, where it acts as a reagent or prepares other nickel compounds.
Weighing the Risks and Looking Ahead
As much as industries get good use from nickelous acetate, it brings challenges. Health risks come up where workers breathe nickel compounds over long periods, possibly leading to breathing problems or allergic reactions. Wastewater from factories carries dissolved nickel, which could find its way into rivers and soil. These risks push for better ways to handle, reuse, or replace this compound.
Some companies recycle nickel from used plating baths, cutting down on waste. A few researchers study less toxic alternatives, pushing for similar performance without the safety risks. Still, nickelous acetate proves tough to beat for now, mainly because its chemistry fits industrial needs so well.
Practical Solutions for a Safer Future
Better ventilation, closed systems, and protective gear protect workers. Smarter waste treatment filters nickel out of water before it hits the environment. I’ve seen more manufacturers invest in monitoring after tighter rules made them rethink their setups. Success comes from regular checks and training, not just new tech.
Nickelous Acetate's Place in Daily Life
For most folks, awareness goes a long way—knowing where basic household objects get their staying power or shine often means trusting a complex supply chain. Nickelous acetate helps tie everything together, from the shimmer of new coins to the toughness of basic tools. A little knowledge on what’s behind the metalwork helps make better choices, whether for work, hobbies, or the environment.
Understanding the Substance
Nickelous acetate, known widely in industry and laboratories, often finds work in electroplating, textile dyeing, and as a chemical reagent. The way this compound looks—green crystals, fairly soluble in water—doesn’t immediately give away the risks packed inside it.
Personal Experience with Nickel Compounds
I’ve handled a range of nickel compounds during projects on metal finishing and corrosion prevention. Even when following lab protocols, the concerns about breathing in dust or touching solutions were clear. Gloves, goggles, and proper ventilation never felt optional. Back in the early days of my studies, peers used to skip protective gear when rushing. Those moments became cautionary tales. One classmate developed a rash after accidental skin contact with a nickel solution—proof of how easily nickel can provoke allergic reactions. These allergic responses stack up—the United States Department of Health and Human Services points out nickel as one of the most common triggers for allergic dermatitis in workers worldwide.
Health Risks Associated with Nickelous Acetate
People exposed to nickelous acetate through the air or through skin might experience irritation. The real worry, though, comes from long-term or high-level exposure. Nickel compounds get tagged as carcinogens. The International Agency for Research on Cancer (IARC) puts certain nickel compounds in Group 1—meaning there’s clear cancer risk with regular, significant exposure. Processes like electroplating—where steam or mist in the air carries nickel particles—raise this danger for workers who don’t stick to strict safety rules.
Short-term contact brings its own set of challenges. Aside from skin rashes, inhalation of dust can lead to coughing, shortness of breath, or worse—persistent lung irritation. Nickel salts can also be toxic to organs when swallowed, though accidents like this are rare in a professional setting with good controls. Repeated exposure increases the risk, and it doesn’t take much for someone to become sensitized for life.
Nickelous Acetate in the Environment
This compound can move into the environment from rinse water or improper waste handling. Nickel ions don’t vanish—they may build up in water or soil, posing risk to aquatic life and plants. The EPA includes nickel in its list of priority pollutants. Once it’s in the water, it doesn’t just harm wildlife; there are stories about community agriculture taking a hit when water sources became tainted. Nickel accumulates in plants grown in contaminated soil, showing up in crops that people and animals eat. Small factories in towns sometimes overlook best disposal practices, letting nickel waste seep into drains—a persistent issue for environmental health.
Keeping Risks in Check
Workplaces can drive down risks with a steady commitment to protective equipment, well-maintained ventilation, and regular air monitoring. Simple habits—washing hands, changing out of work clothes, never eating in the workspace—keep nickel dust from making it home. For communities, clear guidelines about dumping and runoff, plus routine water testing, stand as practical safeguards. More training and easy access to information about chemical safety save both human health and the ecosystem from needless harm. Every nickel compound brings a price, but with firm attention and respect, that cost needn’t come at the expense of worker wellbeing or the land around us.
Understanding Nickelous Acetate
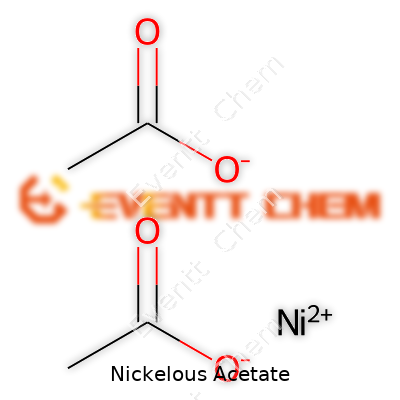
Nickelous acetate, also known as nickel(II) acetate, shows up in chemistry labs and factories in a green, crystalline form. The chemical formula for this compound is Ni(CH3COO)2. Most often, you’ll find it as a tetrahydrate, which means the full formula reads Ni(CH3COO)2·4H2O. Each nickel ion in this salt pairs with two acetate ions and four water molecules. That composition isn’t just some trivia—it shapes how the compound behaves and what it gets used for.
Why Knowledge of the Formula Matters
Chemists and industry workers care about the right formula for nickelous acetate because a misstep can mess up results or even risk safety. The balance of nickel and acetate ions, along with the water of crystallization, drives how the compound dissolves, reacts, and deposits in processes like electroplating. If you’ve ever seen old coins cleaned up or metal tools given new life with a shiny, corrosion-resistant surface, nickelous acetate played a part behind the scenes. A strong grip on the formula avoids mistakes, keeps experiments on track, and gives predictable results that workers can count on.
Real-World Uses Bring Real-World Challenges
Nickelous acetate gets used in labs to prepare catalysts, in textile dying, and especially for nickel plating. People expect smooth coatings and bright finishes. Getting this needs careful ratio control; too much water in the mix or a swapped ion can ruin the final metal layer. Workers have told stories of batch failures traced back to an off-formulation salt. For people handling heavy machinery or electronics, this kind of issue becomes more than an academic misstep—it costs time, energy, and money. A clear formula, Ni(CH3COO)2·4H2O, helps prevent headaches all around.
Health and Environmental Concerns
With industrial chemicals, safety never takes a back seat. Nickelous acetate doesn’t rate as harmless. Nickel compounds, including this one, can cause skin sensitization. I’ve seen colleagues who broke out in rashes after forgetting a glove for just a few minutes. Nickel dust and solutions shouldn’t be breathed in or touched for long periods, since chronic exposure links to more serious health concerns. Teams I’ve worked with have adopted strict labeling, clear documentation, and up-to-date Material Safety Data Sheets to keep everyone informed.
Beyond individual safety, disposal of nickel compounds demands attention. Nickelous acetate, if dumped down the drain, enters waterways and threatens marine life. Rules in most countries require treatment or special disposal. Waste management staff often need refresher courses. No one wants to risk fines or environmental harm due to a simple misunderstanding about what’s in a compound or what its formula actually means for handling.
Supporting High-Quality Work and Responsible Use
Reliable, accurate chemistry underpins more than just theoretical work. Whether plating a bumper in an auto shop or running a university experiment, knowing the exact composition ensures precision and keeps people safe. Experienced workers recommend double-checking labels and keeping a sample for archive, not out of distrust, but as insurance against production hiccups.
The chemical formula for nickelous acetate—Ni(CH3COO)2·4H2O—is more than text in a handbook. It’s a key piece of practical knowledge that shapes reactions, safety measures, and environmental responsibility. Accurate information and ongoing training make the difference between smooth operations and problems no one wants to face.
Recognizing the Risks
I’ve spent a good chunk of my career in labs and warehouse settings where chemicals like nickelous acetate show up on inventory sheets. Storing it without enough thought leads to risks folks rarely see until something goes wrong. This salt contains nickel, which health agencies link to skin allergies, asthma, and cancer with long-term exposure. Spills or mishandling don’t just threaten workspace safety; they pose environmental headaches.
Choosing the Right Container
Nickelous acetate often comes as a green, crystalline powder. It draws in moisture easily and dissolves in water. Standard protocol points to airtight containers, made from glass or high-density polyethylene. Metal cans react, so those never come off the shelf for this job. My own small lab once learned the hard way—using an unlined steel drum ruined a shipment and ruined the container.
Keeping It Quiet and Dry
Humidity brings more problems than most expect. Nickelous acetate clumps fast, and spills become harder to clean up. Dry storage space helps avoid that mess. Temperature swings don’t do much good either. Room temperature feels ideal—nothing fancy, just steady and cool, with no direct sunlight.
Staying Away from Unwanted Company
No matter how much space saved, never tuck nickelous acetate beside acids, strong oxidizers, or reducing agents. Even a sealed bag in the wrong spot triggers headaches—unwanted reactions, fire risk, and sometimes gas releases. Years of sorting shelves taught me the value of chemical segregation. Good labeling and lockable cabinets stop mistakes from new hands in the storeroom.
Supporting Health and Safety
Every workplace keeps safety data sheets on-hand for chemicals like this one. Rely on them: they spell out legal storage and handling steps. Legislators don't dream up these policies for fun; they follow patterns from past workplace incidents. Clear safety labeling, spill kits, and enough PPE ready nearby prevent costly downtime. Respirators and nitrile gloves work well—nobody wants a rash or lung trouble from dust exposure.
Environmental Care Starts in the Storeroom
It’s tempting to cut corners with storage, especially when inventory pressure runs high. Those of us with stories of cleanup learn that shortcut costs arrive later. Nickelous acetate belongs away from drains and never gets poured down the sink. Store it atop leak-proof trays or bins to catch spills before they leave your locker or closet. Broken containers find their match with a secondary one underneath, not only for peace of mind but also to answer for environmental audits by regulators. Sewage treatment plants don’t break down nickel compounds. If even a kilo ends up outside—years of soil or water damage result.
Training and Routine Checks
No safety protocol works if nobody follows up. Training new staff, labeling shelves in plain English, and running spot inspection drills help everyone stay sharp. Keep audit logs, refresh expiry dates, and rotate older stock to the front. Step-by-step, these habits stop surprises. In every lab I’ve seen run well, the best safeguard comes from people who see the value in learning from those who handled these chemicals before.
Tough Love for Safe Storage
Nickelous acetate never belongs in a forgotten corner or in makeshift bins. It isn’t fancy tech or a distant science; it’s simply responsibility—protecting your team, local water sources, and your business from preventable harm. Common sense blends with up-to-date science here, and experience turns policy into second nature. That’s the real measure of safe storage.
Understanding Risks and Why They Matter
Nickelous acetate doesn’t always make headlines, but it shows up in labs, plating shops, and research facilities across the world. Splash it on your hands, and skin rashes or irritation show up soon after. Breathe in dust or fumes, and lungs can suffer. Anyone who spends time with metal salts knows that safety demands more than gloves and a mask sitting on the bench. I’ve seen folks brush off safety notes, only to deal with an itchy arm or worse—nosebleeds after working with open containers in a cramped room. Risks grow if you cut corners.
Personal Protective Equipment Isn’t Optional
I once worked in a plating lab where we all wore nitrile gloves, goggles with side protection, closed-toe shoes, and lab aprons. Gloves mattered most—years of chem lab experience taught me how easily skin absorbs nickel compounds. Latex won’t cut it with nickel because of possible chemical penetration. Goggles mostly kept my eyes safe when dust or small droplets splashed. If you spill any, you wash up fast—no delays.
Good Air Matters as Much as Gloves
Ventilation sometimes gets overlooked. I’ve set up fume hoods and simple local exhausts to keep airborne particles away from people’s faces. Nickelous acetate dust or fumes become a big issue in small rooms or when heating up solutions. In my experience, shops using decent air extraction saw fewer complaints about headaches, chest tightness, or fatigue. If fume hoods aren’t practical, open windows and fans help—anything to stop stale, dusty air from building up.
Storage Keeps Trouble Away
Nickelous acetate always sat far from acids, strong bases, and food in my workspace. Chemical-proof containers with solid lids stopped leaks and cross-contamination. Labeling means more than neatness—it keeps the wrong people, especially new hires or students, from accidental exposure. I’ve caught more than one coworker grabbing the wrong bottle, so labels always stood out, readable from a distance.
Cautious Waste Disposal Makes a Difference
Some folks pour chemical waste straight down the drain. This causes trouble. Nickel compounds harm aquatic life, and local rules ban dumping nickel salt solutions or rinsings like regular waste. We always used sealed waste containers marked for heavy metal collection, then arranged for hazardous waste pickup through certified handlers. Small steps like this keep drinking water and soil safer for everyone nearby.
Training Turns Rules Into Habits
Written protocols look good on the wall, but habits develop from solid training and repeated reminders. In busy workplaces, people skip steps unless they’re reminded and supervisors set the example. We ran short refreshers each month, sharing real stories about close calls and cleanup drills. True learning happened in those moments—the difference between reading about nickel dermatitis and seeing a coworker’s rash pop up out of nowhere.
Everyday Choices Make Labs Safer
Working with nickelous acetate never became routine for us. Over time, it gets easier to remember why we wear gloves, why we don’t store acids next to metal salts, why we vent the room, and why training matters. The right approach saves skin, lungs, and even jobs in the long run. People new to the lab world quickly realize these aren’t just rules—they’re the lessons learned the hard way, passed down so fewer people have to learn on their own skin.

| Names | |
| Preferred IUPAC name | Nickel(II) acetate |
| Other names |
Nickel(II) acetate
Nickel diacetate Nickel acetate Acetic acid, nickel(2+) salt |
| Pronunciation | /ˈnɪk.ləs əˈsiː.teɪt/ |
| Identifiers | |
| CAS Number | 373-02-4 |
| Beilstein Reference | 3537445 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1233477 |
| ChemSpider | 12718 |
| DrugBank | DB14486 |
| ECHA InfoCard | ECHA InfoCard: 100.028.052 |
| EC Number | 209-170-2 |
| Gmelin Reference | Gmelin Reference: 3248 |
| KEGG | C01780 |
| MeSH | D009537 |
| PubChem CID | 8891 |
| RTECS number | QR6125000 |
| UNII | ZU9X0248M1 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Ni(CH3COO)2 |
| Molar mass | 248.84 g/mol |
| Appearance | Light green crystalline solid |
| Odor | Odorless |
| Density | 1.78 g/cm3 |
| Solubility in water | Freely soluble |
| log P | -1.26 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.8 |
| Basicity (pKb) | 5.68 |
| Magnetic susceptibility (χ) | +792.0e-6 cm³/mol |
| Refractive index (nD) | 1.529 |
| Dipole moment | 5.5 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 96.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -877.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1507.7 kJ/mol |
| Pharmacology | |
| ATC code | V03AB61 |
| Hazards | |
| Main hazards | May cause cancer; harmful if swallowed, inhaled, or absorbed through skin; causes skin and respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H317, H334, H340, H350, H360D, H372, H410 |
| Precautionary statements | P210, P261, P273, P280, P301+P312, P302+P352, P304+P340, P308+P313, P330, P332+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2 2 0 |
| Autoignition temperature | > 316 °C (601 °F; 589 K) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 oral rat 350 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 105 mg/kg |
| NIOSH | QR3500000 |
| PEL (Permissible) | 1 mg/m3 |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 250 mg/m3 |
| Related compounds | |
| Related compounds |
Nickel(II) nitrate
Nickel(II) chloride Nickel(II) carbonate Nickel(II) oxalate |



