Lead Acetate: From Historic Curiosity to Modern Caution
Historical Development
People have worked with lead compounds for a long time. Lead acetate, sometimes called “sugar of lead,” has attracted attention since the days of the Roman Empire. Romans loved sweetening wine with this material, unaware of the risks. European apothecaries stocked it for centuries, counting it as a useful ingredient in dyeing, printing, and even cosmetics. Over time, scientific understanding pulled back the curtain on its true nature, revealing a double-edged sword: striking utility but undeniable danger. The wake-up call arrived as researchers connected lead exposure with public health disasters, prompting tighter restrictions across the globe.
Product Overview
Lead acetate today mostly turns up in chemistry labs, hair dye formulations, or industrial processes. The substance usually appears as white or colorless crystals. It draws water from the air, so makers and users handle it under dry conditions and keep it in sealed containers. Commercial lead acetate exists in two forms: the trihydrate (Pb(CH3COO)2·3H2O) and the anhydrous version, each with different handling protocols due to varying water content.
Physical & Chemical Properties
Lead acetate dissolves with ease in water and glycerin, giving rise to clear solutions. Its sweet taste once led to accidental and intentional poisonings, especially before regulators stepped in. Melting begins at 80°C for the trihydrate form, and the dry compound decomposes at hotter temperatures. While not flammable, lead acetate breaks down into less-desirable lead oxides and acetic acid during heating. Unchecked exposure to heat, moisture, or acids leads to a cocktail of new compounds—sometimes with even more serious hazards.
Technical Specifications & Labeling
A bag or bottle of this material always carries warning labels. Regulatory authorities enforce strict labeling, including hazard pictograms for acute toxicity and health risks. Typical purity for research or industrial-grade lead acetate falls above 98%, with trace-metal impurities limited by detailed standards. The chemical formula and batch information stay front and center for supply chain traceability. Packaging keeps both users and the environment in mind, relying on leak-proof, tamper-evident seals to keep lead dust inside. Handling guidelines come with every shipment, pointing out the use of gloves, goggles, and—crucially—ventilation.
Preparation Method
The classic preparation method starts with lead(II) oxide or metallic lead reacting with acetic acid. Producers combine the lead source and acetic acid, often heating gently to trigger the reaction. Reaction vessels keep air exposure to a minimum, limiting oxidation of acetate ions. The mixture gets filtered and purified several times, then allowed to cool, encouraging large crystals to form. These crystals undergo drying in vacuum ovens—since allowing them to sit in a humid environment turns the substance gummy and risks contamination. Each step demands careful control. Wastewaters and residues containing lead stay separated and get processed as hazardous materials to stop further environmental harm.
Chemical Reactions & Modifications
Lead acetate acts as a useful reagent in organic synthesis, serving both as a source of lead ions and as an oxidizing agent. Chemists tap into its ability to precipitate sulfates, chromates, and other anions to figure out which compounds are present in a sample. In old-school dyeing or printing, mordanting reactions anchor dyes to natural fibers, sometimes deepening colors and making them more fade-resistant. Modifications, such as forming basic lead acetates or lead subacetates, enhance stability or create unique binding properties. Yet every new derivative stirs up fresh questions: each must be tested for toxicity, solubility, and durability before anyone feels comfortable using it in a real-world setting.
Synonyms & Product Names
Across different industries and over time, lead acetate has acquired several names. “Sugar of lead” comes from its deceptive sweetness, while “plumbous acetate” or “lead(II) acetate” flag the metallic roots and oxidation state. Chemists sometimes refer to it as Goulard’s Powder or Goulard’s Extract, names inherited from the 18th-century French surgeon Thomas Goulard, who used diluted solutions for topical medical treatments—practices long abandoned as knowledge of toxicity grew. On suppliers’ catalogs, you also find “Lead diacetate” or variations showing hydration, such as “Lead acetate trihydrate.”
Safety & Operational Standards
Anyone working with lead acetate grapples with strict regulations and safety protocols. Every worksite using this compound must provide dedicated ventilation systems, air monitoring, and thorough employee safety training. Handlers suit up in chemical-resistant gloves, lab coats, and protective eyewear. Occasional spills or residues call for special lead-specific cleaning agents—never household bleach or ammonia, which might stir dangerous fumes. Waste goes straight into lined containers marked for hazardous materials recycling. Regulations, like OSHA’s permissible exposure limits in the United States, set the maximum allowed lead dust or vapor in the air, targeting long-term worker health. No one takes shortcuts: ignoring safety can mean lifelong health impacts for individuals and legal trouble for companies.
Application Area
A shrinking list of applications holds onto lead acetate. The hair dye industry remains one of the last major users, especially for gradual color restorers aimed at men’s grey hair. Only a handful of countries still allow this, and regulations grow tighter every year. Chemists turn to it for laboratory analysis, using its ability to form insoluble salts with various anions as a diagnostic tool. Lead acetate sometimes appears in specialized electroplating or printing operations, but substitutes are taking over as awareness of environmental impacts grows. Textile dying and leather tanning, once major consumers, have mostly phased it out in favor of less-toxic compounds.
Research & Development
The story of lead acetate in research doesn’t focus just on new applications: much of it revolves around phasing the material out. Scientists probe its interactions with biological systems, seeking not only to understand health effects but also to unravel ways to remove traces from drinking water or soil. Research into alternatives for industrial and laboratory purposes builds steam; every new molecule synthesized has to match lead acetate’s useful properties without copying its toxicity. Academic groups and manufacturers invest resources into green chemistry approaches that avoid or neutralize hazardous byproducts. Many of these efforts reveal opportunities—novel chelating agents for detection or remediation, biodegradable replacements for dye mordants, or less-toxic additives for lab work.
Toxicity Research
Nothing about lead acetate’s usefulness can overshadow its danger. Decades of studies link lead exposure with everything from neurological damage and developmental delays in children to kidney failure and reproductive harm in adults. The substance crosses the placental barrier and starts accumulating in bones, passing along from parent to child. Lead acetate solutions once treated burns and skin diseases—now, most medical professionals recoil at the thought. Animal studies echo the same message: as levels rise, nervous systems falter, organs break down, and lifespans shrink. Global bans on lead acetate in food or cosmetics reflect the consensus forged by this avalanche of scientific evidence. Testing protocols follow standardized routes, demanding careful blood tests, tissue analysis, and chronic exposure monitoring. Only airtight procedures protect lab workers and users alike.
Future Prospects
Lead acetate’s future narrows, shaped by rising health consciousness and stricter regulatory frameworks. As countries tighten policies and public awareness grows, replacements keep taking root. Green chemistry efforts may soon wipe out lead-based mordants in textiles and phase out analytical uses in favor of safer detection reagents. Hair color formulations already race to adapt; whatever comes next must offer performance without health drawbacks. In environmental sectors, work to filter and trap lead ions from contaminated water sources keeps lead acetate’s removal in focus. Looking ahead, lead acetate serves as a story of changing attitudes: once valued for its sweet taste and flexible chemistry, now studied mostly as a cautionary tale. The compound may stick around in the annals of chemistry textbooks and toxicology case studies, but its days as a household or industrial mainstay look numbered.
What’s Lead Acetate Doing in Our World?
Some chemicals stick around for a reason, even when we have our doubts about them. Lead acetate is one of those substances. For decades, people have turned to it for making and fixing things. Painters, textile workers, and even men looking to darken their hair have all run into this compound at one point or another.
From Dyes to Detection: Industry Relies on Lead Acetate
Lead acetate’s reputation in the factory still stands strong. Manufacturers use it as a fixative in dyes and paints. Without it, certain pigments just won’t hold their color as well, especially in textiles. Leather tanners mix it in to give hides just the right shade.
Scientists also use lead acetate in labs. It reacts in predictable ways, making it useful to detect things like hydrogen sulfide gas—giving a quick thumbs up or down in quality control tests. Some types of batteries have relied on it as well, drawing on its chemistry to improve conductivity or performance.
Personal Care and the Dark Side of History
Stories about lead acetate show up in home bathrooms, mostly from people looking to touch up graying hair. It anchored some formulas for men’s hair dye products, especially those that slowly darken hair to look more “distinguished.” After years of common use, the risks outweighed the rewards, sparking crackdowns by governments. For instance, in the United States, the FDA banned lead acetate from hair dye products by October 2022 after health experts sounded alarms about the danger of lead exposure.
Lead is toxic. That’s just a fact, learned the hard way after generations fell sick from lead-based water pipes, paint, or even pottery. The body stores lead in bones, and the effects can show up slowly: headaches, stomach pain, and worse problems if exposure continues. Kids face the greatest danger. Their bodies absorb more, and their brains react strongly to lead. Even the CDC says no amount of lead in blood is safe.
A Question of Safety: Time to Find Alternatives
Keeping lead acetate around isn’t worth the gamble for most people. Modern science gives us better—and safer—ways to color leather, dye fabric, or test for gas leaks. Many companies have already moved on, choosing less dangerous alternatives, even if they cost more or take some trial and error to get right. In my own family, we check labels and keep away from older bottles or products that could still use lead salts. Educating others on these dangers can make a difference, too.
Governments and businesses have a duty to keep workers, consumers, and the environment safe. Routine checks, updated regulations, and honest product labeling all play a part. Some places still use lead acetate in factories or for specific research jobs, but oversight and careful handling should always apply. The world moves forward when we lean on safer chemistry and transparent science.
Clean, safe innovation beats tradition every time—especially when health is on the line. Lead acetate may have earned its place in history, but that doesn’t mean it deserves a spot in our future.
Lead Acetate Isn’t Just Chemistry—It’s a Health Threat
Most folks only hear about lead when there’s a crisis—contaminated water in Flint, peeling paint in old homes, tainted soil near highways. Lead acetate might get less attention, but it deserves a closer look. This compound used to sneak its way into hair dyes, a relic from the days when men colored beards or mustaches with “Grecian Formula.” That old-fashioned bottle promised to turn grey black, but it delivered something far riskier.
What Science Says About Lead Acetate
Lead is toxic to humans. There isn’t a safe level of exposure. We’ve known for decades that this heavy metal causes nerve damage, lowers IQ in kids, and builds up in bones until it starts affecting organs. The Centers for Disease Control and Prevention (CDC) sets a blood lead reference value at 3.5 micrograms per deciliter for children, and the recommended level for adults is not much higher. Lead acetate puts that all at risk. It’s water-soluble, absorbs through skin, and can get into the bloodstream from dyes, cosmetics, or old industrial uses.
There is nowhere for lead to “belong” in the body. Any amount is too much. The National Toxicology Program calls lead acetate “reasonably anticipated to be a human carcinogen.” The American Academy of Pediatrics points to evidence that even low levels of exposure can change the way a child’s brain develops. Many doctors who spent years treating kids with mysterious learning disabilities or delayed speech have identified lead exposure as a silent culprit. I’ve seen how one overlooked source—an old “safe” product—can turn a family’s world upside down.
Regulatory Gaps Kept the Door Open
The U.S. Food and Drug Administration waited until 2022 to ban lead acetate from hair dyes, much later than countries in Europe and Canada. For years, some manufacturers claimed the chemical remained on hair and didn’t reach the skin. Yet lab results and patient experience proved otherwise. Regulatory gaps let products linger on shelves, even as safe alternatives existed. People who used these products often never suspected a risk because labels rarely gave a plain warning.
The bigger issue runs deeper than hair dye. Lead acetate found its way into other industrial processes: printing, textiles, lab reagents. Workers faced exposure in cramped factories or labs, sometimes without any warning or protective gear. All this happens while medical experts push for zero exposure and governments drag their feet on updating old chemical laws.
What Protects Us: Information and Action
Parents want their children to grow up healthy. Nobody chooses to expose themselves or their loved ones to a neurotoxin. If you suspect you’ve come into contact with a material like lead acetate, ask your doctor for a simple blood lead test—it’s a low-cost tool that can catch exposure early. Get rid of old bottles of dyes, avoid imported cosmetics lacking clear ingredient stickers, and check workplace safety protocols. Share concerns with community health boards. Companies need to reformulate products or add warning labels—not in five years, but now.
Staying safe means listening to scientific findings, advocating for safer consumer products, and not turning a blind eye to risks hidden by tradition or outdated laws. Lead acetate may seem technical or old-fashioned, but its danger is very real wherever it sticks around.
Understanding the Risks
Lead acetate shows up in older hair dyes, some labs, and less frequently, in industry. You can spot its powder, usually white or sometimes slightly sweet-tasting—though tasting this stuff isn’t something anybody should do. What matters is recognizing that even small amounts can harm the nervous system, hit the kidneys hard, and cause long-term health problems like anemia or developmental delays in children. Getting the basics on how to protect yourself might seem obvious, but in practice, people can get lax. That can invite disaster.
Personal Protective Equipment Isn’t a Luxury
You pick up a container of lead acetate and skin touches the powder. That’s all it takes for absorption to start. Gloves, goggles, and lab coats cut down the risk right at the source. I’ve known people who think a quick job means gloves aren’t needed. Later, they find dust on their hands—sometimes someone’s already rubbed an eye. Latex or nitrile gloves, tucked under the cuffs, make a huge difference. Respirators, not just dust masks, matter if there’s any risk of inhaling powder or fumes. Cheap safety glasses keep particles from making contact with eyes.
I’ve worked jobs where corners tried to get cut, especially on hot summer days when sweating inside a lab coat felt like torture. Still, the equipment feels preferable to shaking for weeks with symptoms nobody wants.
Keep Your Space Clean
Lead dust doesn’t just land on your skin—it can settle on benches, molded corners, even favorite coffee mugs. Good safety involves dedicated workspaces and regular cleaning, using damp cloths or specialized vacuums with HEPA filters. Dry sweeping just kicks up dust. Handwashing before leaving the room can’t get skipped. Food and drinks stay outside the workspace, and anything brought in accidentally, like gum or snacks, can sit collecting contamination.
At one site I worked, a technician forgot these simple rules—he ended up with fine white dust on his lunch, and after weeks with headaches, an exam tied it to low-dose lead exposure.
Proper Storage and Waste Disposal
Lead acetate belongs in sealed, labeled containers, dried and kept away from heat or sunlight. A lockable chemical cabinet with clear signage is a must. I’ve heard stories of children finding old bottles, thinking they were candy (the powder tastes sweet—one reason it’s dangerous). Good organizations educate workers with signs and clear written protocols, and keep inventories up to date.
Every workplace should have a system for disposing of contaminated gloves, wipes, and clothing. It’s not the same as regular garbage. Separate hazardous bins and close-by washing stations save everyone trouble later. I once witnessed clothing tossed in regular laundry, only to contaminate a coworker’s child later on.
Education and Vigilance
A lot of safety comes down to people knowing what they’re dealing with. MSDS (Material Safety Data Sheets) need to be within reach, not buried under forms. Quick training sessions, even thirty minutes, can remind everyone of symptoms and emergency steps. Blood lead testing, especially for people working with this chemical often, should be regular—annual at minimum. Spotting early signs helps prevent problems getting out of hand.
Safer Choices Exist
Plenty of labs have started replacing lead acetate with less toxic alternatives. If there’s any way to switch the process, it’s worth considering. It’s not grandstanding—just looking out for everyone, from workers to families. We all depend on each other to get home safe.
Understanding Lead Acetate
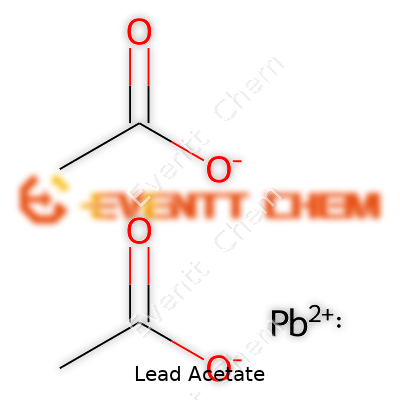
Lead acetate carries the chemical formula Pb(C2H3O2)2, or simply Pb(CH3COO)2. This compound hides an unsettling history, from Victorian hair dye to industrial uses. Most people come across lead as a threat, and rightfully so. In fact, recognizing chemical formulas such as this isn't about solving a puzzle—it's about making sense of the risks woven into old remedies, decaying plumbing, and some manufacturing.
Why Lead Acetate Matters Right Now
Anyone working around old paint, imported cosmetics, or even antiquated pipes could benefit from knowing the face of lead compounds. Lead acetate carries a sweet taste, which seems innocent—yet that sweetness masks heavy toxicity. In the 1800s, it played a role in fake wines, and more recently, it turned up in traditional hair straighteners. The sweet taste isn’t just a historical anecdote; it explains why unintentional poisonings occurred. These stories give the chemical formula a context that textbooks rarely show.
Public Health Meets Personal Caution
Sometimes I wonder if the public truly connects the dots between formulas on a label and the threat behind them. Agencies like the CDC have hammered home that lead, in any form, attacks the nervous system and impairs development, especially in young children. Exposure never happens in isolation—peeling paint in old apartments, water leaching from outdated pipes, and imported products sneak these threats into daily life. The trouble is, a line like Pb(CH3COO)2 doesn’t scare people on its own. It should.
Back in college, my chemistry professor spilled a vial with this compound during a demonstration. He paused the lesson, not to scold or frighten, but to describe just how little it takes to hurt a child—and how easy it is to overlook a formula. That stuck with me. The smallest mistakes can have big consequences.
Unpacking the Formula
Imagine the formula as a map. Pb signals lead, a toxic heavy metal. (C2H3O2) stands for the acetate group, which by itself comes from vinegar. Together, they make a salt—a little too at home in the powder jars of old barbershops or research labs. Sometimes chemistry tries to mask danger with fancy notation, but the risks don’t smooth out just because a label looks technical.
Solutions: Awareness, Action, and Alternatives
Banning compounds like lead acetate from cosmetics and dye products signals progress. California and the European Union have both outlawed its use in personal care items. On the front line, schools and parents ought to get familiar with these names. If a product label spits out Pb(CH3COO)2, treat it as a warning, not a curiosity.
Testing kits aren’t out of reach for teachers or renters, and they can catch contamination before it causes harm. Pushing industry to innovate with nontoxic dyes or additives, instead of relying on history’s shortcuts, helps stop the cycle. And every time a chemical formula like this one becomes household knowledge, the world gets a little safer.
Understanding Lead Acetate and Its Hazards
I remember the first time I heard about lead acetate in a college chemistry lab. The instructor started by spelling out its poison symbol and launching into a lecture about old-school uses. Ancient Romans used it as a sweetener. That story sticks with me because it proves an essential point — just because something has been around for ages doesn’t mean it’s safe to use, buy, or sell today.
People sometimes look for chemicals like lead acetate out of curiosity, for home experiments, or for specialized old recipes found in antique books. But the truth is, modern science has shown just how dangerous this compound can be. According to the CDC, exposure to lead damages the nervous system, kidneys, and reproductive system. Children are particularly vulnerable, and lead can linger in the body for years. With stakes this high, it’s not a surprise that access to lead acetate is tightly controlled.
Laws, Regulations, and Why Shop Policies Changed
Finding a store that offers lead acetate online or in-person has become close to impossible for private individuals. Chemical retailers still sell it, but they check credentials and licensing — you can't just add it to a web cart. Pharmacies and hardware shops long ago took it off their shelves, after health authorities and environmental agencies cracked down on toxic consumer products. The U.S. Environmental Protection Agency (EPA), the European Union, and other watchdogs list lead compounds on their risk registers. In the U.S., purchasing lead acetate often requires a business license and a stated industrial, educational, or research reason.
Besides the rules, most suppliers understand the risks of selling toxic substances to the public. Several tragic mass poisonings, usually accidental, prompted lawmakers to clamp down. As a result, sites like Amazon and eBay ban hazardous chemicals like lead acetate — both out of concern for lawsuits and public safety. No responsible company wants a child poisoned after a package lands in the wrong hands.
Dangers of DIY Chemistry and the Need for Better Education
I’ve watched friends get enthused about home science kits. That curiosity is good, but it brings a responsibility to understand what’s safe. The search for something like lead acetate could come from a science fair project or misguided curiosity. Schools and science teachers play a huge role here — they need to explain why some compounds are available and others are absolutely off-limits. Instead of encouraging potentially hazardous purchases, teachers should point students toward accessible, harmless materials that still deliver “wow” moments in science.
What to Do If You Need Lead Acetate for a Legitimate Purpose
Chemists and industry workers who require this substance for specialized processes need to go through proper channels. Start with a university or registered laboratory. Accredited suppliers usually ask for extensive documentation, including precise end uses, safety plans, and disposal arrangements. They often need proof that a buyer understands local laws and has access to proper protective equipment and disposal facilities. For the public, safe disposal of any old bottles of lead acetate should go through hazardous waste facilities, not storm drains or regular trash pickups.
A Better Approach to Chemicals at Home
Most people searching for lead acetate don’t realize what they might get themselves into. It helps to connect the dots with history — seeing how the easy access of the past led to tragedy, and recognizing how modern controls are designed to prevent repeating those mistakes. Hobbyists and students can find safer experiments without risking their health. Government and educational bodies do well to keep supporting public information on chemical dangers, encouraging safe curiosity, and delivering facts plainly, without jargon or scare tactics.
| Names | |
| Preferred IUPAC name | lead(II) ethanoate |
| Other names |
Salt of Saturn
Sugar of Lead Plumbous acetate Acetic acid lead(2+) salt Lead(II) acetate |
| Pronunciation | /ˈliːd ˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 301-04-2 |
| 3D model (JSmol) | ``` leadacetate.mol::/JSmol/3db78dbd-6b8d-4d8b-8ab6-799b560ed9d6 ``` |
| Beilstein Reference | 3539743 |
| ChEBI | CHEBI:7806 |
| ChEMBL | CHEMBL137885 |
| ChemSpider | 5374546 |
| DrugBank | DB14772 |
| ECHA InfoCard | 100.999.624 |
| EC Number | 206-104-4 |
| Gmelin Reference | 778 |
| KEGG | C13947 |
| MeSH | D007854 |
| PubChem CID | 515889 |
| RTECS number | OJ4550000 |
| UNII | 6DRR7G8885 |
| UN number | UN1616 |
| Properties | |
| Chemical formula | Pb(C2H3O2)2 |
| Molar mass | 325.29 g/mol |
| Appearance | White crystalline solid or sometimes colorless crystals |
| Odor | slight acetic odor |
| Density | 2.55 g/cm³ |
| Solubility in water | 44.3 g/100 mL (20 °C) |
| log P | -0.07 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.8 |
| Basicity (pKb) | 9.8 |
| Magnetic susceptibility (χ) | '−47.0×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.622 |
| Viscosity | 1.54 cP (25°C) |
| Dipole moment | 2.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 215.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -667.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1447.0 kJ/mol |
| Pharmacology | |
| ATC code | S01XA13 |
| Hazards | |
| Main hazards | Toxic if swallowed, inhaled, or absorbed through skin; causes damage to organs; suspected of causing cancer; may cause reproductive or developmental effects. |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02, GHS07, GHS08 |
| Signal word | Danger |
| Hazard statements | H302 + H332: Harmful if swallowed or if inhaled. H350: May cause cancer. H360Df: May damage the unborn child. Suspected of damaging fertility. H373: May cause damage to organs through prolonged or repeated exposure. |
| Precautionary statements | P210, P261, P264, P270, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P314, P330, P362+P364, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-2-" |
| Lethal dose or concentration | LD50 oral rat: 466 mg/kg |
| LD50 (median dose) | 466 mg/kg (oral, rat) |
| NIOSH | NL2975000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Lead Acetate: 0.05 mg/m³ (as Pb) |
| REL (Recommended) | 0.05 mg/m³ |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Lead(II) nitrate
Lead(II) chloride Lead(II) sulfate Lead(IV) acetate Lead tetraacetate Lead carbonate |



