Isopropyl Acetate: A Straightforward Look at a Versatile Chemical
Historical Development
Isopropyl acetate has stuck around as a reliable solvent for decades, showing up in chemical catalogs as far back as the early 20th century. Folks in the chemical industry first produced this compound by tackling basic esterification, reacting isopropanol with acetic acid. Early adoption found a stronghold in paint and coatings manufacturing, where rapid evaporation and compatibility with resins mattered. People noticed improvements in applications ranging from lacquers to specialty inks. As demand grew for fast-drying, low-residue solvents, isopropyl acetate gained ground and worked its way into various industrial toolkits.
Product Overview
This ester shows up as a colorless liquid, often described by its sweet, fruity odor that comes off as a lighter version of pear drops. It isn’t something the average person stumbles upon, yet professionals in printing, coatings, and even some flavor and fragrance companies keep it close by. Companies market isopropyl acetate under names like 2-propyl acetate, isopropyl ethanoate, and IPAc. In warehouses and labs, tanks and drums labeled with common trade names line up for blending, formulation, or direct use.
Physical & Chemical Properties
You can spot isopropyl acetate by its boiling point of about 89°C and its melting point, which jumps down to around -73°C. Density clocks in at about 0.87 g/cm³ at room temperature, and it brings a vapor pressure of roughly 24 mmHg at 20°C. Folks appreciate its solid miscibility with common organic solvents like alcohols, ketones, and hydrocarbons, but basic water solubility stays modest, hovering near 3 g/100 mL at 20°C. Flammability stands out. You won’t find anyone recommending open flames or careless handling near this liquid. These facts make it a clear target for industries aiming for quick solvent-removal or cleaning jobs that need a rapid dry without persistent residue.
Technical Specifications & Labeling
Every barrel of isopropyl acetate carries clear designations, from purity levels (usually above 99%) to batch number and manufacturing details. Labeling details include hazard pictograms signaling flammability and warnings against inhalation or direct skin contact. Safety data sheets (SDS) line out thresholds for occupational exposure, list fire-fighting measures, and explain what to do in case of accidental spills or leaks. Transportation stays regulated under UN code 1220, classifying isopropyl acetate as a dangerous good with flammable liquid characteristics. In production facilities or warehouses, it sits far from ignition sources and under ventilation unless sealed tightly.
Preparation Method
Industrial groups stick to acid-catalyzed esterification, using isopropanol and acetic acid at tested ratios and temperatures to maximize yield. Sulfuric acid usually drives the process, ensuring completion. Chemical engineers remove water by distillation to push the equilibrium toward product formation, then separate unreacted raw materials for re-use. The remaining liquid gets dried and distilled, isolating isopropyl acetate at a commercially viable purity, ready for downstream users or further purification. Plants capable of this synthesis range in size, but consistency and yield stand as the two driving metrics for success and profitability.
Chemical Reactions & Modifications
Chemists have played with isopropyl acetate, looking at how it responds under different conditions. Under hydrolysis, it snaps back into isopropanol and acetic acid, an entirely reversible reaction, which holds practical value for recycling or neutralizing waste streams. Oxidation runs into trouble with ester stability; the compound prefers to resist further breakdown unless harsh oxidizers or catalytic conditions force a change. Grignard reagents and strong reducing agents will reduce the ester function, returning the original alcohol. These pathways explain why isopropyl acetate sees limited roles in reactions requiring harsh processing.
Synonyms & Product Names
Industry literature uses quite a few alternate names, such as 2-propyl acetate, isopropyl ethanoate, and IPAc. Over the years, certain trade names have become standard in procurement documents and regulatory filings, making cross-industry communication a little more manageable.
Safety & Operational Standards
Handling isopropyl acetate calls for reliable ventilation, proper storage in flameproof cabinets, and clear labeling to prevent confusion with less volatile liquids. Regulatory groups like OSHA and the European Chemicals Agency have set exposure limits and safety guidelines. Gloves and goggles are non-negotiable in most settings, and anyone working with this material needs training on spill prevention and emergency cleanup. Waste streams head to controlled incineration or recycling facilities to avoid environmental harm. In shipping and storage, containers remain sealed and kept away from oxidizing materials or direct sunlight.
Application Area
Printers and coatings specialists pick isopropyl acetate to thin inks and varnishes, thanks to its evaporation rate and solubility with common resins. Paint removal products leverage its low boiling point for quick drying. Electronics manufacturers use it in specialty cleaning solvents for circuit boards and mechanical parts, appreciating the residue-free finish. Fragrance and flavor formulators slip in a hint for certain fruity notes. Pharmaceutical industries locate a use in extraction and purification, counting on it to partition useful compounds or strip away unwanted byproducts.
Research & Development
Research pushes the boundaries on new extraction methods, greener synthetic routes, and recycling techniques for spent isopropyl acetate. Some labs hustle to optimize catalysts that bring down energy costs or replace sulfuric acid with less corrosive acids. Innovation targets safer process conditions, waste reduction, and routes from renewable feedstocks. Not everyone pays attention to these studies, though in a world cracking down harder on hazardous waste, these tweaks pave the way for more sustainable industrial practices.
Toxicity Research
Toxicologists measure risk by looking at acute and long-term effects. Inhalation causes short-term dizziness, nausea, or headaches at high concentrations, and long-term studies keep an eye on chronic exposure effects—usually finding low systemic toxicity unless doses reach impractical highs. Animal trials have tested reproductive, mutagenic, and developmental impacts, with limited evidence pointing toward major concerns at regulated exposures. Environmental groups watch how isopropyl acetate breaks down in air and water, monitoring for persistent or bioaccumulative breakdown products, though current evidence keeps this risk low.
Future Prospects
Industry shifts toward lower-carbon manufacturing and tighter safety standards push producers to adopt more closed-loop recovery, step up on product stewardship, and integrate alternative catalysts. As regulations tighten and customers ask for more sustainable sourcing, companies invest in renewable isopropanol and acetic acid, aiming for greener labels and lower emissions. Engineers innovate around safer handling systems, improved worker protection, and automated monitoring. Future outlooks predict growing demand in electronics, specialty coatings, and pharmaceutical intermediates, all tied to ongoing pushes for cleaner processes and stricter oversight.
Versatile Solvent in Everyday Products
Many people look past the chemical names in ingredient lists, but isopropyl acetate quietly shows up in plenty of places. At home, it supports the paint thinner in your garage, helps some cleaning products cut through grease better, and gives certain sprays a quick-dry finish. People who work with artificial nail removers probably smell its sweet, fruity fumes from time to time. From my own memory, the constant changes in nail polish remover brands meant some worked quicker than others — turns out, isopropyl acetate is what helped the good ones do the job in seconds. The stuff evaporates fast, so surfaces avoid being sticky or smeared.
Tool for Industry and Manufacturing
Factoring in safety, speed, and cost, factories lean on solvents like isopropyl acetate to blend coatings, create inks, and enable smooth operations on production lines. Printing companies, especially the ones pumping out glossy magazines or packaging, depend on solvents that won’t distort colors or gum up expensive presses. I’ve seen offset printing done the old-fashioned way, and the cleanest prints always came from inks with solvents that didn’t linger or spread.
Beyond inks, isopropyl acetate has a place in formulating some adhesives and coatings. Furniture factories and auto paint shops use it to get that “just-finished” look. Decent evaporation means freshly sprayed items can move down the line without clinging particles — a real bonus during high output weeks.
Role in Fragrance and Flavors
Perfume and flavor makers like isopropyl acetate for its mild scent and safety profile. It offers a fruity note that blends well with more complex fragrances. Though it’s there in small amounts, the sweet, almost pear-like aroma hides bitter flavors and slightly sharp smells in industrial solvents.
In food, it rarely appears as a direct ingredient but sometimes crops up in flavor labs testing complex “blends” before big batches get approval. Food scientists check for purity, making sure none lingers where it shouldn’t, sticking to FDA limits and established safety standards.
How Safety Enters the Picture
Tightly regulated, isopropyl acetate must meet health standards from agencies around the world. Breathing high levels irritates eyes and lungs, so factory workers need solid ventilation and reliable masks. The fruity smell provides some warning, but safe storage practices really separate the responsible companies from careless ones. I remember walking into a shop that cleaned their brushes in a bucket open to the air — bad idea. Living proof that training matters, because chemicals slip through the cracks if no one pays attention.
Room for Better Practices
Good supply chains matter as much as good science. Sourcing isopropyl acetate in a way that respects both people and the planet helps reduce risks in the long run. Stronger labeling rules and hands-on safety training do more to protect workers than just putting a manual on a shelf. Upgrading equipment to keep fumes away from lungs, along with frequent checks for leaks, keeps both efficiency and health in balance. Changing habits, even small ones, can mean safer shops, cleaner homes, and fewer surprises later.
Understanding What’s On Your Workbench
There are not many chemicals out there that pop up in both your nail polish remover and your laboratory solvent shelf, but isopropyl acetate fits that bill. This clear liquid smells a bit fruity, almost like pears, though it can fool you into thinking it's harmless. In most workplaces, isopropyl acetate gets used as a solvent or a flavoring agent—yet, beyond its utility, people rarely notice how easily it catches fire. Flammability isn’t just a label printed in red on a barrel: it’s a risk that follows wherever this chemical lands.
What Flammability Looks Like in the Real World
Experience tells you to be cautious around open flames when pouring gasoline or using alcohol wipes. Isopropyl acetate sits right at home in that league. The flash point for this stuff hovers just under room temperature—about 2°C (36°F)—which means you don’t need much heat before vapors start to ignite. Vapors heavier than air can drift down and find an ignition source, even far from the original spill. Folks who’ve seen solvent fires break out would agree: nothing brings out panic faster than a dancing orange flame where none ought to be.
Why Should You Care?
Letting your guard down around flammable chemicals never ends well. The U.S. National Fire Protection Association reports thousands of industrial fires every year with causes ranging from spilled liquids to electrical sparks. One major warehouse fire took out an entire stockroom because an unsupervised isopropyl acetate spill leaked toward a heater. Fires like those don’t just threaten property or productivity—they can reshape lives in a heartbeat, especially for anyone nearby. There’s no safety net for hindsight.
Common Mistakes and Real Solutions
People sometimes treat familiar chemicals as less dangerous. Someone pouring off a gallon of isopropyl acetate and tossing the rag in a bin, without thinking twice about where it lands—just another Tuesday for some factory workers. Locking up flammable liquids seems obvious, but improper storage and absorbent material bins left uncovered have caused serious trouble. Sprinkler systems and fire extinguishers help, yet they only work if the crew actually knows how to use them.
What works better is a culture of asking questions and double-checking procedures. Teaching everyone—from the operations manager to the janitor—to recognize flammable symbols and read Safety Data Sheets actually moves the needle. Some companies make it standard to run “fire drills” based on chemical spill scenarios, not just old-school office evacuations. These drills cost time but build instincts, so the right reflexes take over during real trouble.
Personal Stories Shape Safety Culture
Someone once told me about a close call in a print shop. Nobody thought much of the isopropyl acetate because it was “just another supply.” Turns out, a spark from static electricity sent an invisible vapor cloud flashing back toward a workstation. The technician smelled burning hair, but the worst was a scorched sleeve and a lesson learned. Folks tightened up after that, even buying better spill kits.
Flammability only sounds like abstract science until you witness a near miss. Simple awareness—knowing what can burn, where fumes go, and how quickly things escalate—keeps everyone out of the emergency room. Isopropyl acetate looks innocent. In reality, it asks for constant respect.
Understanding the Risks
Isopropyl acetate shows up in a lot of places—from making flavors and fragrances to helping with electronics cleaning. It’s got that sweet, fruity smell and makes work easier in certain labs and manufacturing floors. Underneath that pleasant odor, though, it packs a punch. Even small spills can start vaporizing fast, building up in the air, and that’s where stories of headaches, dizziness, or skin rashes begin. If handled carelessly over long periods, these side effects stack up quickly. Respiratory issues are no joke. A crowded workshop with no ventilation turns from busy to risky quickly, especially for workers already dealing with asthma or allergies.
Why Personal Protection Counts
Thick gloves and goggles aren’t an inconvenience. Too many friends in industry admit forgetting them “just for a minute,” only to wind up with irritated eyes or cracked, inflamed hands. Skin absorbs isopropyl acetate readily enough to bring worries for repeated exposures. And in one memorable incident, a loose-fitting glove turned a dropped beaker into hours of stinging pain. Splash protection means something real in these moments. Chemical-resistant gloves—nitrile, not latex—keep you safer. The right goggles, the kind that hug the face, stop accidental splashes from turning into vision-threatening emergencies.
Ventilation and Storage Matter
Good air flow is often the best defense. Fume hoods, open windows, and exhaust fans work together to keep vapors below limits set by bodies like OSHA, which suggests keeping airborne concentrations under 250 ppm. Closed containers seal away the risks once the workday wraps up. I remember how someone left a half-full bottle near a warm window—just a few hours later, that part of the lab reeked, triggering fire alarms and a costly investigation. Isopropyl acetate is flammable—one stray spark or even a hot light can go bad quickly. No open flames, no smoking, and no shortcuts around storing containers in flame-proof cabinets.
Spills and Fire: Quick Actions Save the Day
Most spill stories sound the same—nobody expects them, and that’s why being prepared cuts down on panic. Keeping spill kits right next to the work area goes a long way. Absorbent pads, sand, or vermiculite will stop a puddle from spreading. Wiping up with paper towels just creates more hazards. Fire extinguishers rated for flammable liquids belong close at hand, not locked away in another room. One small mistake—knocking over a flask, forgetting to ground a drum—reminds everyone that even brief slips get expensive and dangerous fast.
Training and Culture Build Better Habits
Isopropyl acetate safety needs regular reminders—on-the-job talks, clear labels, and simple checklists. Nobody should need to guess about where the emergency shower sits or how to handle an eye wash station. From my own time in shared labs, the teams that talked safety every week had fewer problems. Strong habits, built on hands-on training and respectful reminders, cut down on short cuts and “almost mistakes.” Respect for this chemical makes workplaces stronger for everyone, not just the safety officer or supervisor.
Smart Substitution and Engineering Controls
Leaning into safer alternatives where possible makes sense. Some operations swap isopropyl acetate for less volatile solvents if the process allows. Automated transfer systems cut down on fumes and spills, letting machines do the riskiest pouring. Regular checks on ventilation equipment and clear emergency signs turn what could be stressful environments into manageable ones.
Isopropyl Acetate: More Than Just a Formula
Most people won’t recognize the name right away, but isopropyl acetate — with the chemical formula C5H10O2 — shows up quietly in industries, products, and sometimes even on the labels of things found in garages and workshops. This compound forms from a simple reaction between isopropanol and acetic acid, joining the ranks of esters that play big roles in chemistry and manufacturing. It comes with a smell that reminds some folks of pears or even bananas, which hints at its use in flavoring and fragrance, but the story stretches much further.
Real-World Applications and the Science Behind Them
In the paint shop or the print facility, solvents like isopropyl acetate work to break down sticky residues or dissolve inks that just won’t budge with soap. Chemists favor it for its balance — enough volatility for it to evaporate without leaving behind a gummy mess, and enough strength to handle stubborn substances.
It doesn’t just show up in labs. The nail polish remover sitting in a purse, the cleaning agent handy under a sink, or the flavoring agent adding something special to a soda — isopropyl acetate touches all of these. That reach gives the formula real significance. It’s more than just numbers and letters; it’s a building block of the things people use every day.
Safety and Environmental Considerations
Just because a compound is common doesn’t mean all risks are understood. Breathing in the vapors from isopropyl acetate can annoy the nose and throat, and overexposure sometimes leads to dizziness. Flammable liquids like this call for real respect, especially around heat or open flame. Keeping up with safety training and using the right protective equipment matters in any workplace that handles it. Home users don’t always get that kind of instruction, so clear warnings on the label make a difference.
As for the environment, fast-evaporating solvents contribute to air quality problems if vented without controls. Companies and regulators keep a close watch, setting limits on emissions and exploring greener solvent options. Responsible disposal, tighter packaging, and improved workplace practices already help cut down on spills and waste.
The Push for Responsible Chemistry
Transparency stands out as a necessary value in the modern chemical industry. Consumers want to know about the ingredients in products. Governments and watchdog groups push for honest descriptions, tracking systems, and better public outreach. Companies that work with isopropyl acetate and other chemicals often respond by sharing more about sourcing, processing, and disposal — not just to follow the law, but to build confidence with their customers.
Progress never stops. Researchers keep searching for alternatives that deliver similar performance with lower health and environmental costs. Water-based formulations, bio-based solvents, and more careful stewardship each mark a small step in the right direction.
Understanding the Chemistry, Owning the Impact
Memory of cleaning as a kid or standing in an art classroom with that sweet-pungent smell in the air brings home why the formula C5H10O2 means something. Knowing what’s behind a label, what happens after use, and how each step touches people and the planet gives true weight to the answer — it’s not just about science, but about how science fits with responsibility in daily life.
This Stuff Packs a Punch
Isopropyl acetate, with its sharp smell and fire-happy character, always lands on the list of chemicals that demand respect. Anyone working with solvents knows the headache of improper storage. You walk into a storeroom reeking of solvent or see discoloration on containers—those are signs most folks learn to pay attention to early on. Having spent years in labs and factories, safe chemical storage never feels like wasted effort. Small mistakes with volatile organics like isopropyl acetate can spiral fast—from ruined materials and angry inspections to some real safety scares.
Ventilation and Location Matter
Solvents crave cool, breezy spaces. Isopropyl acetate loves to evaporate and mix with air, so always store it away from direct sun or any hot equipment. The best place is where fresh air flows, not in cramped cupboards or utility closets. Fresh air keeps vapors down and makes leaks easier to detect. Most fire codes push for chemical storage outside main workrooms or in specialized cabinets. At one site, I watched a poorly ventilated storeroom fill with vapor. Only a lucky catch avoided an ER visit that day. So many experienced workers have their own stories about what happens when airflow gets overlooked.
Keep Ignition Sources Far Away
Everyone knows tales of accidental sparks and near-disasters. Isopropyl acetate ignites with little encouragement—a static discharge can be enough if the vapor builds up. Always use “no smoking” storage areas. Set up clear, bold signage. Keep the space bolt-clear of anything that sparks or heats up. That means steering clear of room heaters and exposed switches. Many places now use explosion-proof electrical fixtures in solvent storage rooms, and that shift makes a huge difference in reducing risk.
Pick Safe Containers and Label Clearly
Cheap or careless packaging invites trouble. Steel cans with tight-sealing caps do the job well for most operations. I’ve seen sweaty plastic jugs weaken after a summer stuffed next to pipes; over time, that turns into a leak or a break, right in your hands. Original containers almost always have clear hazard labels—big, simple, and color-coded. If you decant isopropyl acetate into smaller containers, always copy over those hazard warnings. In a real emergency, clear labeling buys precious seconds and keeps responders safe.
Organization Isn’t Just for Looks
Busy storerooms get messy fast, and every tech has seen labels drop off or chemical cans stacked tilting on the edge. A solid storage system sets rules about which shelves get high-risk stuff such as isopropyl acetate. Store only what you need, shelve chemicals away from acids and oxidizers, and keep everything organized by compatibility charts. Don’t guess—read the safety data sheet and double-check the latest recommendations. At one plant, dedicated chemical shelves with spill trays beneath them cut cleanups and complaints almost overnight.
Learn from the Real World
Paying attention to details—ventilation, containers, labeling, segregation—keeps people and property safe. Many lessons get learned the hard way. Years ago, a friend overlooked a leaky can, which led to days spent managing the fallout. Investing in airtight practices doesn’t just protect assets; it sends a message that everyone’s safety is worth every minute spent on proper storage. Follow the facts, learn from missteps, and treat chemicals like isopropyl acetate with the respect they demand.
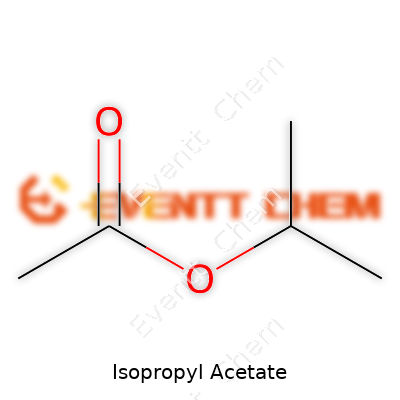
| Names | |
| Preferred IUPAC name | propan-2-yl ethanoate |
| Other names |
2-Acetyloxypropane
Acetic acid, isopropyl ester Isopropyl ethanoate IPAc |
| Pronunciation | /ˌaɪ.səˈproʊ.pɪl ˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 108-21-4 |
| 3D model (JSmol) | `Isopropyl Acetate JSmol string`: ``` CCOC(C)C=O ``` |
| Beilstein Reference | 633873 |
| ChEBI | CHEBI:31210 |
| ChEMBL | CHEMBL31890 |
| ChemSpider | 6826 |
| DrugBank | DB02347 |
| ECHA InfoCard | 100.114.277 |
| EC Number | 203-561-1 |
| Gmelin Reference | 8225 |
| KEGG | C00902 |
| MeSH | D017374 |
| PubChem CID | 8024 |
| RTECS number | NT2020000 |
| UNII | AKT60NDF10 |
| UN number | UN1220 |
| CompTox Dashboard (EPA) | DTXSID3049230 |
| Properties | |
| Chemical formula | C5H10O2 |
| Molar mass | 102.13 g/mol |
| Appearance | Colorless liquid with a pleasant, fruity odor |
| Odor | Fruity |
| Density | 0.87 g/cm³ |
| Solubility in water | 17 g/100 mL (25 °C) |
| log P | 1.30 |
| Vapor pressure | 35 mmHg (20°C) |
| Acidity (pKa) | 14.8 |
| Basicity (pKb) | pKb ≈ -1.78 |
| Magnetic susceptibility (χ) | -51.5e-6 cm³/mol |
| Refractive index (nD) | 1.376 |
| Viscosity | 0.62 cP (20°C) |
| Dipole moment | 2.88 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 282.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -488.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2115.7 kJ/mol |
| Pharmacology | |
| ATC code | D01AE24 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H225, H319, H336 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P233, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | The flash point of Isopropyl Acetate is "2 °C (36 °F)". |
| Autoignition temperature | 460 °C |
| Explosive limits | 1.3–7.1% |
| Lethal dose or concentration | LCLo (rat): 12,000 ppm/4H; LD50 (oral, rat): 4,830 mg/kg; LD50 (dermal, rabbit): 17,000 mg/kg |
| LD50 (median dose) | 6,750 mg/kg (rat, oral) |
| NIOSH | NIOSH: NT3675000 |
| PEL (Permissible) | 250 ppm (980 mg/m³) |
| REL (Recommended) | 250 ppm (950 mg/m3) |
| IDLH (Immediate danger) | 2000 ppm |
| Related compounds | |
| Related compounds |
Acetic anhydride
Isopropyl alcohol Propyl acetate Ethyl acetate Methyl acetate |



