Isobutyl Acetate: Deep-Dive Commentary On a Vital Industrial Solvent
Historical Development
Before big refineries and advanced organic synthesis, manufacturers relied on fermentation and the byproducts of alcohol distillation to get their hands on isobutyl acetate. Chemists noticed its fruity aroma early on, using steamed wood shavings soaked in acetic acid and isobutanol to yield the ester. Over time, as the chemical industry grew after World War II, larger plants started scaling up production using Fischer esterification. Modern methods keep streamlining yield and purity, but the original concept—a straightforward acid and alcohol reaction—still guides production. This blend of tradition and innovation means isobutyl acetate remains a staple in industries that prize both performance and reliability.
Product Overview
Anyone who has handled paints, inks, or coatings probably encountered isobutyl acetate without realizing it. Its stamp shows up wherever a volatile solvent is needed to deliver fast drying, crisp application, or a hint of fruitiness in scents and flavors. The product comes as a clear, colorless, mobile liquid with a telltale sweet, fruity odor. Companies sell it in drums or totes, and it fits easily into manufacturing streams. As an ester, its ease of production and low toxicity compared to aromatics gave it staying power in a shifting regulatory landscape.
Physical & Chemical Properties
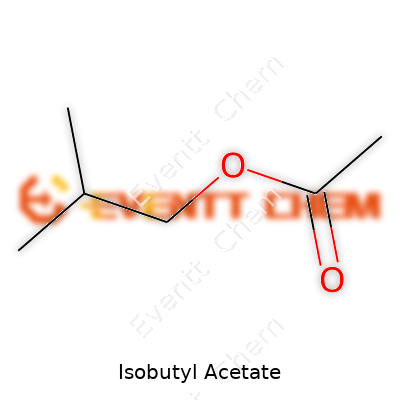
Isobutyl acetate carries the formula C6H12O2. On the bench, its density clocks in at about 0.87 g/cm3, and it boils at 117°C—making it more volatile than water but easy to handle on standard equipment. It’s less dense than water, resists dissolving in it, and flashes at around 22°C, so care around ignition sources matters. That familiar fruity smell has made it a flavor favorite, but the same volatility that gives aroma also mandates airtight storage. Its solubility in organic solvents like alcohol and ether makes it a workhorse in mixed systems that need speed, like ink drying or paint spraying.
Technical Specifications & Labeling
Producers label drums of isobutyl acetate with the UN number 1220 for shipping and customs. Safety data sheets flag the main metrics: purity usually surpasses 99%, water content stays below 0.1%, and acidity must remain in trace amounts to avoid corroding equipment. Color appears almost clear, a sign of proper distillation. Labels warn of flammability and direct users to keep the containers cool and tightly closed. Information panels break down manufacturer batch and test data because quality control underpins safe blending and consistent output. The smell may catch attention, but those numbers on a label keep labs in compliance and plants accident-free.
Preparation Method
On an industrial scale, most plants use Fischer esterification, mixing isobutanol and acetic acid in the presence of a sulphuric acid catalyst. Water sweeps out as a by-product, and distillation purifies the batch. Some players lean on continuous flow reactors for better yield and waste reduction. The guiding principle is simple: generate a steady stream of ester, keep the acid concentration optimal, and scrub out any water and excess reactant. Smaller labs and educational settings often repeat this reaction in glassware, boiling up small batches to demonstrate the reaction’s efficiency and the appeal of its fruity product.
Chemical Reactions & Modifications
Isobutyl acetate holds steady under most benign conditions, but strong acids or bases can rip the ester bond apart back into isobutanol and acetic acid. Under other routes, nitration or chlorination yields functionalized esters for specialty applications. Those in polymer chemistry sometimes use it as a reactant for grafting side chains onto larger molecules, tuning the final product’s volatility or flexibility. Its moderate reactivity makes it a handy solvent, able to avoid side reactions during mixing or curing, which sets it apart from more aggressive chemicals.
Synonyms & Product Names
On labels and in catalogs, isobutyl acetate answers to several names: 2-methylpropyl acetate, 1-propyl acetate, and isobutyl ethanoate. In trade circles, buyers see names like IBAC, iso-butyl ethanoate, or various brand names tied to distributor codes. Whatever the alias, it always keeps that same set of recognizable properties: a fruity aroma, quick evaporation, and suitability where a less hazardous solvent fits the bill.
Safety & Operational Standards
Working with isobutyl acetate requires eye protection, gloves, and plenty of ventilation. Occupational health agencies like OSHA set exposure limits around 150 ppm for 8-hour shifts to stave off headaches or respiratory irritation. In storage and transportation, handling protocols focus on fire risk—operators ground drums, use explosion-proof pumps, and label everything clearly. Regular leak checks and spill response plans mean mishaps seldom escalate. Reputable suppliers offer training on safe handling and urge proper PPE, backed by detailed data sheets that flag the main routes of exposure: inhalation, skin contact, or accidental ingestion (rare in industry, more likely with unsafe practices).
Application Area
Coatings and paints see the lion’s share of isobutyl acetate. It speeds up drying, thins formulations, and helps pigments flow smoothly onto surfaces with minimal brush marks. The printing industry embraces it for fast-setting inks. Manufacturers of adhesives, cleaning agents, and nail polish blend it for its solvent strength and pleasant residual odor. Food and flavor houses value it as an additive that punches up fruity profiles in confectionery or beverages. Because it bridges several industries, isobutyl acetate shows up almost anywhere a quick-drying, low-toxicity solvent matters, and substitutes for harsher or more odiferous alternatives like toluene.
Research & Development
The push for greener chemistry drives a lot of new research. Teams look at biobased pathways, fermenting sugars into isobutanol, then esterifying it with bio-acetic acid, slashing the carbon footprint. Efforts in analytical chemistry keep finding lower-impact routes for detecting trace solvent residues in consumer goods. Researchers trial new catalysts and process tweaks to maximize yield while using less energy and water. In aerospace and advanced coatings, teams keep studying the solubility parameters and balance between evaporation rate and finish quality to dial in next-generation formulas.
Toxicity Research
Most evidence points to isobutyl acetate as a low-toxicity solvent, but occupational exposure still requires care. Animal studies show mild central nervous system effects at high doses. Inhalation over the long term occasionally causes mild liver or kidney stress, but not at levels seen in modern plants with engineering controls. It breaks down in the environment fairly quickly, but regulators keep tabs on ambient levels near plants to avoid ecosystem disturbances, especially in water. As part of REACH and TSCA, producers share comprehensive toxicology data to assure downstream users and regulators alike. Improved monitoring helps head off exposure long before levels reach a point of concern in workspaces.
Future Prospects
Regulatory pressure and changing consumer habits signal a transition toward bio-based and lower-emission isobutyl acetate. Plants in Brazil and the US explore bioethanol-based production to offset fossil inputs and shave lifecycle emissions. Waterborne coatings and new adhesives are expanding its footprint, especially as regulations push out solvents with tougher air quality impacts. As global demand for electronics, auto refinishing, and green building materials grows, demand for safer, easily handled solvents increases. Ongoing research will keep bolstering the safety, sustainability, and versatility of isobutyl acetate, ensuring it plays a part in the next chapter of chemical manufacturing.
A Familiar Scent in Daily Life
Step into a room just after someone finishes painting, and a fruity, sweet smell fills the air. That often comes from isobutyl acetate, an organic compound found in many common products. The scent can surprise people, as it carries a simple reminder—chemistry reaches far beyond labs and textbooks. Apple and pear notes in perfumes and flavors often use isobutyl acetate. And every so often, someone will bite into a processed candy or baked treat and find themselves catching a trace of it on their tongue. It’s not there by accident.
Core Ingredient in Solvents
Factories and workshops often use isobutyl acetate for its power as a solvent. People working with lacquer and varnish brushes depend on it to keep wood finishes smooth and streak-free. The chemical dissolves resins and oils, so professional painters and those in furniture restoration count on it for projects. Quick drying paints need solvents like this, as they help bind all the pigment and binder together and leave no sticky mess behind. Nail polish removers also draw on its effectiveness, which helps break down tough polishes in seconds.
Flavor and Fragrance Industry
The reach of this compound stretches into foods and fragrances. Isobutyl acetate brings a burst of fruity notes to candies, snacks, and sodas. In the world’s flavor houses, technicians tweak the levels to match the juicy, fresh notes in pineapple and raspberry. It might surprise some, but even natural fruit aromas get an extra bump from this compound, especially after processing knocks out many original scents.
Perfume creators also work with isobutyl acetate for its versatility. It helps create long-lasting apple, pear, and floral bouquets. Unlike some bitter chemical smells, this compound blends well and is gentle on the senses at safe concentrations.
Industrial and Household Uses
Cleaning supplies draw on isobutyl acetate as well. Glass cleaners and degreasers often list it among their main ingredients, since it cuts through oily grime while leaving fewer streaks than water-based solutions. This feature comes in handy—from car repair shops wiping down tools to busy kitchens scrubbing countertop stains.
It also plays a role in plastics and adhesives. Some plastics get their flexibility in part from isobutyl acetate, which helps improve texture and workability. Adhesive manufacturers tap into its dissolving properties, enabling them to create products for everything from paper crafts to automotive assembly.
Safety and Environmental Considerations
Exposure to large amounts can cause sore throats, dizziness, or headaches, so proper ventilation stays important in workshops and factories. Government safety agencies provide clear exposure limits for workers. Long-term studies still keep testing for cumulative effects, as demand for solvents rises in manufacturing. That’s why workplace training and personal protective equipment remain critical.
Disposal also deserves attention. Releases into water sources can threaten aquatic life, since isobutyl acetate doesn’t break down instantly. Most developed countries enforce rules around storage, spill response, and emissions. Innovations in green chemistry now push for safer replacements or recycling practices, so industries can reduce their impact while meeting consumer needs.
Toward Smarter Use
People tend to underestimate how many products rely on this one compound for their texture, smell, or cleaning power. That puts some responsibility on producers and regulators to keep checks in place for safety and sustainability. Switching to less volatile or biodegradable alternatives makes sense where possible, without forcing companies to overhaul well-tested processes overnight. Continued research gives everyone—from the paint mixer to the average shopper—better tools for smarter, safer use.
Understanding Isobutyl Acetate in Everyday Use
Walk through a paint shop or step into a room freshly coated with lacquer, and it’s possible you’ll catch a whiff of isobutyl acetate. This clear liquid pops up in all sorts of workplaces, from furniture factories to nail salons, and even hides inside fruity flavors and perfumes. Many folks don’t suspect a thing about the chemistry behind those glossy finishes and sweet scents. Knowing what’s in the air matters, especially for anyone rolling up their sleeves with these products day in and day out.
What Makes Isobutyl Acetate Useful?
Isobutyl acetate does a solid job dissolving paints, resins, and oils. Its fruity aroma also earns it a job inside artificial flavors. Technicians like how quickly it evaporates, leaving surfaces ready for the next step. Because of this, the chemical spreads into the air fast—sometimes faster than we might want. This property helps when quick work is needed, but it also means the fumes build up easily in enclosed spaces.
Risks in Handling
I’ve found that people just starting out with paints and solvents might shrug off the idea that a clear liquid like isobutyl acetate could do harm. Yet studies show even a few minutes of high-level exposure can bring on dizzy spells, throat dryness, or eye irritation. My hands have tingled and my nose felt raw after a rushed cleanup job with poor ventilation. Safety data from the CDC agree: inhaling large amounts of this solvent can bother the lungs, acting much like other chemical fumes on the jobsite. There’s a reason both OSHA and the CDC set workplace exposure limits.
Skin absorbs liquids faster than many expect. Without gloves, that light tingling in the fingers can grow into redness and itching if spills happen often. Splash a bit in the eyes and there’s real discomfort. Scientists call this an irritant effect—it doesn’t burn through skin, but enough contact will remind anyone of the risks. Long-term studies don’t tie isobutyl acetate to cancer, though any chemical that triggers headaches or nausea needs attention.
Solutions for Safer Handling
From years helping friends rehab old homes, I’ve seen the difference that open windows or a strong fan make. Air movement cuts down fume levels, keeping headaches and throat burns at bay. Simple changes like wearing chemical-resistant gloves, eye protection, and an apron have kept my skin free of rashes, even through hours of careful brushwork. If reusable gloves aren’t handy, even disposable nitrile ones do better than bare hands. Reading product labels and checking the latest safety fact sheet, which almost always lives online these days, makes a big difference in spotting trouble before work starts.
Leaving lids off containers and letting rags soak in open buckets can fill a room with more vapors than folks guess. Small steps—closing lids, sealing up waste and storing chemicals away from living spaces—make a bigger difference than expensive gear most of the time. Training new workers or DIYers helps, too, since many injuries come from folks taking shortcuts or skipping safety gear out of habit.
Treating Safety with Respect
Isobutyl acetate doesn’t come with a skull and crossbones on the bottle, but that shouldn’t lead anyone into ignoring its risks. Respecting the simple rules—plenty of fresh air, gloves for every mix or cleanup, goggles for splashy jobs—protects workers, hobbyists, and even curious kids wandering through the garage. In my experience, those small moments of caution keep the chemical useful on the job, without inviting any health surprises later on.
Getting to Know Isobutyl Acetate
Isobutyl acetate stands out for a bunch of good reasons. The first thing that always hits me is the smell. You notice its fruity aroma, almost like pear drops, in everything from markers to cheap perfumes to nail polish remover. This smell comes naturally from the molecule’s structure—an ester combining isobutanol and acetic acid. Its scent isn’t just for show; it signals its role across industries, from flavors and fragrances to solvents.
Evaporation and Volatility
Working with paints, I’ve learned that evaporation rates change everything about how a finish lays down. Isobutyl acetate flashes off at just the right speed. It “dries” quick enough for industrial settings, making it easier for painters and manufacturers to control product appearance. With a boiling point near 118°C (about 244°F), it sits in a sweet spot: not as volatile as ethyl acetate, safer and less irritating than acetone.
Solubility and Compatibility
Isobutyl acetate dissolves a wide range of resins and polymers. I’ve used it with nitrocellulose lacquers and found that it thins them well, without breaking down plastics or finishes. It handles oils, waxes, gums, and some inks; it barely mixes with water, so there’s less worry about humidity causing separation or cloudiness in certain products.
Low Toxicity and Human Tolerance
Spending time in workshops, you start to pay attention to what gives you headaches or skin issues. Compared to stronger solvents, isobutyl acetate doesn’t feel harsh on skin or lungs. Safety data puts it in a lower hazard category, though nobody should drop personal protection. Its lower toxicity supports its use in flavors and food packaging, provided amounts don’t go over regulated limits. Agencies like the FDA keep a watchful eye here, which gives some peace of mind.
Environmental Concerns and Solutions
Production of isobutyl acetate uses petrochemical feedstocks, which means the environmental footprint isn’t small. Spills could threaten aquatic life because it's toxic to fish. The right move involves strict containment, recycling practices, and better ventilation in workplaces. Companies making big batches should switch to closed-systems, to keep emissions down and workers safe.
Recently, “green chemistry” methods have started popping up in research. I've read about bio-based fermentations, using renewable corn or sugar feedstocks to get the same product. Shifting to these methods won’t solve everything overnight, but they point in the right direction—cutting down reliance on oil and lowering pollution risks.
Everyday Use and Safety
From model airplane glue to nail polish removers, isobutyl acetate is everywhere. In my own projects, it has worked well because it gets results without overpowering fumes, and it washes out of brushes with little trouble. Using gloves and keeping a space vented always makes the difference, and I wouldn’t skip those steps just because it’s considered milder than some alternatives.
Anyone working regularly with this solvent—artists, factory workers, hobbyists—should watch for dizziness or nausea. That’s your body’s way of flagging overexposure. Local exhaust fans and simple gloves help keep problems away. Regulations get stricter every year, nudging both companies and users to think ahead about safer workspaces and greener chemistry.
Isobutyl Acetate Can Be Trouble if Stored Carelessly
Ask anyone who’s ever worked in a paint shop or a chemical warehouse, and they’ll tell you storing Isobutyl Acetate isn’t the sort of thing you can do on autopilot. One tiny leak can fill a room with fumes or even spark a costly fire. This isn’t overcautious; it comes from seeing dozens of emergency calls in facilities that overlooked basic safety steps. Even though Isobutyl Acetate seems tame in a tightly capped bottle, its low flash point and flammable vapors change the game completely. Once, I watched an entire shelf of solvents drenched by a burst pipe bring in battalions of cleanup crews, just because the storage room didn’t have proper containment.
Ventilation Isn’t an Afterthought
No one forgets the sharp, fruity smell of Isobutyl Acetate. Without strong ventilation, that odor fills lungs and headaches soon follow. Companies sometimes stash drums in windowless corners to save space—not realizing poor airflow lets vapor levels climb toward dangerous heights. I’ve seen a small workshop lose two days of work because workers felt dizzy and nauseous. Open up those vents and keep air moving. It’s the easiest fix to keep staff from getting sick and reduce the chance of an explosive situation. Most accidents happen not from big mistakes, but from ignoring these small steps until it’s almost too late.
A Cool, Dry Spot Beats Fancy Technology
Temperature makes a bigger difference than most folks expect. One hot afternoon can push barrels close to their bursting point. Storing Isobutyl Acetate far from sunlight and steam pipes saves money and keeps accidents out of the headlines. Warehouses that stick to the basics—solid racks, steady temperatures, low humidity—see fewer leaks and barely ever call the fire department. Chemical compatibility also comes up: Isobutyl Acetate doesn’t get along with oxidizers or acids. A supervisor once lined up incompatible barrels right next to each other, and a small spill made for hours of mop-up and one angry fire marshal.
Containers Matter More Than Labels
Steel or HDPE drums with tight seals keep this solvent in check. Never put trust in borrowed containers or old bottles with cracked caps. I remember a neighbor storing leftover solvent in a plastic jug from the grocery store—by the weekend, it had softened and leaked right through the garage floor. A spill kit should always live close by, filled with absorbents suited for organic solvents. Quick thinking and the right gear have turned near disasters into simple footnotes in many shops I’ve visited.
Training Beats Fancy Policies
Rules on a clipboard don’t count once an alarm rings. Most safe warehouses have one secret: Staff know what’s inside every barrel and how to react. Having team members run through real spill drills pays off far more than another binder of standard operating procedures. The people on the ground pick up on small leaks and catch bad smells right away, long before electronic monitors or inspectors walk in. OSHA records show fewer incidents at sites with routine drills; knowledge translates into quicker responses, smaller spills, and safer outcomes.
Simple Steps Save More Than Money
Safe storage never happens by luck. Good habits, practical training, and the right containers stand between a normal day and a long, costly shutdown. Isobutyl Acetate brings plenty of value to industries, but safety starts with the way it’s stored—well-ventilated, cool, and kept far from anything it shouldn’t touch. I’ve seen it done right, and seen what goes wrong. It comes down to a real commitment, not another checklist. And that makes all the difference where it counts.
Why Flammability Matters
I’ve walked through a lot of workspaces in my life—factories, labs, high school chemistry rooms. Flammable chemicals aren’t exotic rarities. At first glance, isobutyl acetate looks and smells kind of innocent. It’s in fruits and flavorings, pops up in nail polish and sometimes helps plants mimic natural scents. It’s common enough that folks might forget a simple fact: this liquid can catch fire easily.
Straight Talk on the Risk
Isobutyl acetate lights up at around 74°F (23°C). That's low. If a warehouse manager overlooks a spill or leaves a drum open near an exposed circuit, trouble comes quick. In my early days working in an industrial setting, I watched a safety drill where a chemical storage room filled with vapors from a leaky container. One spark could have been all it took.
Fire codes flag isobutyl acetate for a reason. Its vapor spreads silently across a room, and it’s heavier than air, which causes the fumes to travel along floors and collect in low spots. A forgotten pilot light or static electricity from synthetic clothing can provide the ignition. This isn’t paranoia. Industrial accident records show over and over that flammable solvent fumes lead to real fires and explosions.
The Science and Safety Angle
Isobutyl acetate burns with a nearly invisible flame. That makes it even more sneaky. Spraying perfume or using lacquer at home or work doesn’t seem all that risky until you remember the open window, the candle, the cigarette left in an ashtray. More than once, I’ve sat with people who thought a little cross-ventilation would be enough. It isn’t. The vapor lingers and wants to catch.
Authorities like the U.S. National Fire Protection Association and the Chemical Safety Board keep pushing this point. They classify isobutyl acetate as a Class IB flammable liquid. Its flash point sits lower than room temperature, and its vapor mixes well with air, forming an explosive cloud if given half a chance.
Experience and Prevention
In training sessions, I always pressed the rule: store containers tightly closed, far from heat or sparks, and only in places designed for flammable chemicals. There’s no substitution for proper ventilation and fire extinguishers placed within reach. I’ve worked with people who trusted duct tape to patch spills or used plastic trash cans as "temporary" storage. Those shortcuts get people hurt.
At home or at work, respect for the stuff counts more than anything. Purchase only what’s truly needed, rotate stock so old cans don’t build up pressure, teach folks about static electricity, and don’t let complacency creep into routine. Reports from insurance and fire departments mention isobutyl acetate’s role in fires more than anyone should accept. Everyone remembers the spectacular disasters, but most losses come from the day-to-day: a dropped rag, an overheated tool, a sunny spot next to a window.
Better Habits Make All the Difference
The safest workers I know double-check their labels, maintain their storage and treat every flammable liquid like an accident waiting to happen. That mindset saves lives. Isobutyl acetate does its job in manufacturing, painting, flavoring, and perfuming—but always with respect paid to its fire risk. Training, good storage, and some healthy suspicion make working with this solvent much less dangerous, whether you’re in a chemical plant or blending scents at a small shop.
| Names | |
| Preferred IUPAC name | 2-methylpropyl ethanoate |
| Other names |
Isobutyl ethanoate
2-Methylpropyl acetate Acetic acid isobutyl ester |
| Pronunciation | /ˌaɪsoʊˈbjuːtɪl əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 110-19-0 |
| 3D model (JSmol) | `Isobutyl Acetate JSmol 3D model string`: ``` CCCC(=O)OC(C)C ``` |
| Beilstein Reference | 635873 |
| ChEBI | CHEBI:31241 |
| ChEMBL | CHEMBL14335 |
| ChemSpider | 7192 |
| DrugBank | DB14185 |
| ECHA InfoCard | 100.112.230 |
| EC Number | EC 203-745-1 |
| Gmelin Reference | 6079 |
| KEGG | C09702 |
| MeSH | D000693 |
| PubChem CID | 8021 |
| RTECS number | NI3320000 |
| UNII | Z8IJ2JQN2X |
| UN number | UN1213 |
| CompTox Dashboard (EPA) | DTXSID2020967 |
| Properties | |
| Chemical formula | C6H12O2 |
| Molar mass | 116.16 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Fruity |
| Density | 0.871 g/cm³ |
| Solubility in water | 0.7 g/100 mL (20 °C) |
| log P | 1.78 |
| Vapor pressure | 11.1 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 25 |
| Basicity (pKb) | pKb = 10.48 |
| Magnetic susceptibility (χ) | -52.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.394 |
| Viscosity | 1.0 cP (20°C) |
| Dipole moment | 1.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 237.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -428.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4498.7 kJ/mol |
| Pharmacology | |
| ATC code | There is no ATC code for Isobutyl Acetate. |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Autoignition temperature | 427 °C |
| Explosive limits | 1.3–7.5% |
| Lethal dose or concentration | LD50 oral rat 13,400 mg/kg |
| LD50 (median dose) | 6,750 mg/kg (rat, oral) |
| NIOSH | NA0162 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Isobutyl Acetate is "150 ppm (710 mg/m³) |
| REL (Recommended) | 150 mg/m³ |
| IDLH (Immediate danger) | 1600 ppm |
| Related compounds | |
| Related compounds |
Acetic acid
Isobutanol Isopropyl acetate n-Butyl acetate Ethyl acetate |



