Chloroacetyl Chloride: A Deep Dive
Historical Development
In the late 19th century, chemists working with acyl chlorides started to uncover routes for creating reactive intermediates. Chloroacetyl chloride found its niche thanks to its high reactivity, especially in growing pharmaceutical and dye industries looking for more efficient synthesis pathways. As laboratorial techniques and chemical engineering improved over the next century, this compound emerged as a vital building block, fueling growth in agriculture, pharmaceuticals, and the production of specialty chemicals. Those early syntheses, often fraught with handling dangers, forced a gradual shift toward safer, more controlled processes. Some of the most impactful chemical regulations took shape to control intermediates just like chloroacetyl chloride. Now, producers rely on both tradition and advanced protocols, reflecting decades of industrial experience and regulatory pushback.
Product Overview
Producers often view chloroacetyl chloride as an essential intermediate rather than a finished product. Its use covers a wide spectrum, such as preparing herbicides, pharmaceuticals, and dyes. Many industries rely on its selective reactivity to introduce the chloroacetyl functional group—something that opens doors to tailored products that perform consistently. Even as demands change, especially for safer and more sustainable choices, this compound keeps showing up in specialized applications where its unique profile shines.
Physical & Chemical Properties
Chloroacetyl chloride appears as a colorless to pale yellow liquid at room temperature, with a sharp pungent odor and fumes that quickly catch the back of your throat if not handled correctly. Its boiling point sits around 105°C, but it releases corrosive hydrochloric acid vapors long before reaching that temperature. Often, it gets stored in tightly sealed glass or metal containers in dark, cool places to reduce risks of decomposition or uncontrolled reaction. Chemically, this molecule reacts with water almost instantly, releasing heat and dangerous fumes, making it a strong acylating agent, especially useful in organic syntheses.
Technical Specifications & Labeling
Each drum or bottle comes marked with hazard pictograms for corrosivity and environmental harm, not just as a regulatory box-ticking exercise but because small mistakes can spiral into real emergencies. Typical specifications list purity above 99%, with trace reporting for typical impurities like chloroacetic acid and phosgene. Certificates of analysis accompany each shipment, with batch numbers for traceability in case of recall or incident. Regulators mandate clear hazard statements, first aid guidance, and storage instructions, with safety data sheets covering every step of the journey from warehouse to workbench.
Preparation Method
The standard process for producing chloroacetyl chloride relies on reacting chloroacetic acid with thionyl chloride or phosphorus trichloride. In most cases, the process generates byproducts like sulfur dioxide or phosphorus oxychloride, creating a need for robust waste management. Modern plants invest in scrubbers and closed systems to minimize worker exposure and environmental contamination. Some facilities, especially in countries with strict regulations, monitor emissions in real-time and recycle byproduct gases when possible, reflecting a practical approach to tackling both efficiency and community safety concerns. Producers try to balance yield, purity, and the mounting costs of hazardous waste disposal.
Chemical Reactions & Modifications
In a synthetic chemist’s toolbox, chloroacetyl chloride commonly serves for acylation of amines, alcohols, and aromatic compounds. Its reactivity with nucleophiles generates products like chloroacetamides and esters, which in turn help build antibiotics, anesthetics, and agrochemicals. Its straightforward conversion makes it a go-to ingredient when you need to tack on a chloroacetyl moiety. For more advanced chemistry, it acts as a stepping-stone toward heterocyclic compounds, tying into the manufacture of active pharmaceutical ingredients. Given its incompatibility with water and strong bases, chemists keep it well separated from anything moist or alkaline.
Synonyms & Product Names
Chloroacetyl chloride often gets shipped and spoken about under a range of alternative names: Acetyl chloride, chloro-, 2-chloroacetyl chloride, and monochloroacetyl chloride. In chemical catalogs, the CAS number serves as the universal identifier, avoiding mix-ups across languages and labeling regimes. No matter the synonym, veteran users pay more attention to purity grades and supplier reputations than the exact product name stamped on the drum.
Safety & Operational Standards
Direct contact with chloroacetyl chloride spells disaster for skin, eyes, and lungs. Proper training and strict protocols—double-gloving, chemical splash goggles, local exhaust ventilation, and emergency showers—reduce but do not erase the risk. Transporters rely on UN safety codes and specific packaging rules. Emergency services drill spill response and containment procedures, knowing that even small leaks can escalate quickly if vapors spread or reach water sources. On site, workers always have neutralizing agents on hand and wear respiratory protection in any space with poor airflow. Sites that neglect routine safety audits or cut corners on PPE put lives at risk and lay themselves open to harsh regulatory action.
Application Area
Chloroacetyl chloride does much of its work behind the scenes, which keeps many consumers from ever hearing its name. Herbicide manufacturers use it to create compounds that help farmers manage weeds while preserving crops. Pharmaceutical chemists depend on it to build intermediate molecules for antibiotics, painkillers, and specialized antifungal agents. Paint and dye makers value its capacity to introduce new colorfastness technologies. In the lab, I once worked with a synthesis where a chloroacetyl group increased yield and selectivity of an antifungal molecule—proof that sometimes, the right intermediate unlocks performance that more benign alternatives just cannot match. Its role feels indispensable in modern industrial and research chemistry, but few outside these fields recognize its contributions.
Research & Development
Academic and corporate researchers continue to seek less hazardous and more sustainable acylating agents, but chloroacetyl chloride retains a firm grip on applications where alternatives fall short. New developments include continuous-flow synthesis setups, which offer improved control and reduce human exposure during dangerous steps. Some research teams have published greener synthesis routes using recyclable catalysts, helping shrink waste streams and greenhouse gas footprints. In technology-driven fields, computational chemists model reaction pathways involving chloroacetyl chloride, searching for shortcuts and safer substitutes. Still, the compound remains vital for developing new active ingredients for modern medicine and crop science. R&D budgets often reflect the ongoing cost of safety and environmental controls, not a transition away from this chemical’s core role.
Toxicity Research
Animal studies show strong irritancy and corrosive effects from exposure to chloroacetyl chloride, particularly through inhalation or skin contact. Inhaled fumes damage airway linings and can trigger lung edema. Repeated exposure sometimes leads to chronic respiratory conditions and skin sensitization, raising alarms among industrial hygienists and occupational doctors. Toxicology researchers note its rapid hydrolysis to chloroacetic acid and hydrochloric acid in moist environments, amplifying local tissue damage. While high-profile fatal accidents are rare, the chemical’s reputation for danger is well earned and keeps safety officers vigilant. Medical teams stationed at chemical plants regard the first minutes of spill response as critical for preventing long-term injuries, making basic education and regular first-aid drills non-negotiable.
Future Prospects
Demand for chloroacetyl chloride faces pressure from tightening regulations and a growing push toward green chemistry. Producers are investing in cleaner, closed-loop systems to curb emissions and reduce on-site hazards, with some pilot plants exploring enzymatic or solvent-free acylation as possible alternatives. In my experience, the cost and difficulty of replacing such a reactive intermediate with less hazardous compounds often outweighs the perceived benefits—for now. Scientists racing to invent new synthesis pathways face the same balance of performance, cost, and risk that shaped early discoveries. As environmental standards rise and new regulations take hold, manufacturers bank on technical fixes to keep this compound available for critical applications, especially in pharmaceuticals and specialty agrochemicals. The chemical’s long-standing versatility and reliability will likely keep it in the industrial toolkit for years, even as the world continues asking for safer and more sustainable options.
Where Chloroacetyl Chloride Shows Up
Chloroacetyl chloride brings to mind that sharp smell from chem labs most folks try to forget. Its sharp bite and fumes aren’t for the faint-hearted. Industry keeps it busy, mostly in the background, where few see its true effect. Paints, synthetic fibers, and even some medicines owe their existence to reactions sparked by this chemical.
It often marks the start of a chain reaction, leading to the creation of herbicides like alachlor and butachlor. These protect crops from unwanted weeds, keeping food prices manageable. In the world of pharmaceuticals, certain antibiotics rely on chloroacetyl chloride to kick off a reaction step. Without it, some treatments would cost more or never reach the shelves.
Costs and Dangers for People and the Environment
My first memory of handling this stuff sticks out: Two sets of gloves, eye shield, and standing by a large fume hood. Even a drop on skin burns. Breathing its vapor can land someone in the hospital. Sadly, accidents happen. Headlines tell of leaks from plants and injuries when safety rules get pushed aside. The risks don’t stop at the door. Once released, it reacts in the air, producing toxic gases. Nearby towns worry about spills—a real fear, based on cases in India and the US.
Farmers in many countries rely on the herbicides and pesticides made using this chemical. Yet, widespread use of these products feeds debates about health and pollution. Some studies point to water sources near large farms—downstream rivers and wells—where residue has been detected, raising questions for both local governments and consumers. When we see cancer rates or breathing problems spike near chemical plants, folks look for a link. Policy debates kick off, weighing the need for affordable food and jobs against environmental and health concerns.
What Drives Demand, What Shapes Supply
Chloroacetyl chloride isn’t something you’ll find on a supermarket shelf, yet its price moves markets. Shortages in production echo down the supply line, raising the price of crop protection chemicals. Droughts and floods drive up demand for herbicides, as farmers try to make every acre count. In a normal year, global factories keep a steady rhythm. Disruptions—from plant shutdowns in China to stricter rules in Europe—cause waves, sometimes leaving rural stores without supplies.
Despite its value, stricter rules from agencies like the EPA or the European Chemicals Agency aim to limit risks. This means companies pay more for safety measures and sometimes move operations outside strict regions, chasing lower costs, leaving neighboring communities uneasy.
Finding a Safer Path Forward
Having walked past fencing that marks off chemical plants, I know one slip-up can lead to years of consequences. Community groups near these facilities now call for tighter controls, real-time air monitoring, and transparent emergency plans. Digital leak detectors and training staff more often help, but older plants struggle to keep up. Some researchers tinker with cleaner reactions and safer replacement chemicals, but progress slows down when costs rise. The search for alternatives in medicine or farming continues, yet change seldom comes quick.
People want affordable food and life-saving drugs, yet shy from chemical risks in their own backyard. Chloroacetyl chloride stands as a reminder of both the power and price of industrial progress. Its future lies in smarter oversight, stricter safety rules, and steady funding for safer solutions. Until then, gloves stay on, and eyes stay open.
What Makes Chloroacetyl Chloride so Concerning?
Most folks outside the lab haven’t heard about chloroacetyl chloride, but it packs a punch when it comes to chemical danger. I’ve handled this stuff in university labs, and every time, the instructors went pale at the mention of its name. Chloroacetyl chloride burns skin, irritates eyes, ruins lungs, and reacts violently with water. That’s not an exaggeration; even the smallest drop in a moist environment creates clouds of hydrochloric acid gas.
Why Taking Every Precaution Matters
I once saw a fellow student splash a tiny amount onto her glove. In seconds, the material degraded, and she winced in pain before the emergency shower did its job. That experience sticks with you. Unlike solvents such as acetone or ethanol, this compound demands respect. Breathing its vapors, even for a moment, can send you to the hospital with chemical burns on your respiratory tract.
Data backs up this seriousness. The National Institute for Occupational Safety and Health (NIOSH) limits exposure to less than 0.05 parts per million in the air. The US Occupational Safety and Health Administration (OSHA) treats accidental spills as emergencies that need full hazmat suits and an immediate evacuation.
Personal Protective Measures in the Lab
Full-body protection matters more here than for most chemicals. Disposable nitrile gloves aren’t enough—thick butyl rubber works best. Standard safety goggles fall short; a face shield stops splashes to the eyes. Forget sleeves and lab coats alone. A full-length chemical apron and closed shoes complete the barrier.
Fume hoods do the heavy lifting. Even with the gear on, I open reagent bottles only under a fume hood with negative pressure. Good ventilation takes away any vapor before you even notice the harsh, choking smell.
Safe Storage and Transport
Storage makes a difference. I never trusted plastic containers with this liquid. Only glass or fluoropolymer-lined bottles stand up to its corrosiveness. Store far from water, alcohol, and even the faintest trace of acids or bases. A locked, ventilated cabinet marked with hazard labels tells everyone that something dangerous hides inside.
Transport between lab benches or rooms happens in secondary containers, like a rigid chemical carrier. Once, someone dropped a bottle directly on the floor, and the glass broke open. The cleanup turned nightmarish—clouds of acid, alarms blaring—which hammered home the need for double containment.
The Importance of Emergency Preparedness
Nobody expects an accident, but they happen. We kept calcium gluconate gel close by for acid burns and practiced eyewash and shower drills every semester. Spill kits with neutralizing agents, absorbent pads, and gas masks sit in arm’s reach.
Anyone near chloroacetyl chloride should know the emergency routine like the back of their hand. Quick action stops a small splash from becoming a trip to the ER. Training can’t wait until after an incident. The right attitude saves skin, lungs, and sometimes lives.
Building a Culture of Responsibility
Handling dangerous chemicals requires more than strong rules. I remember the relief that came from seeing colleagues double-check each other. Peer accountability reinforces safety better than any poster or training video. More experienced workers help newcomers grasp why these precautions matter.
Creating a safety-first mindset builds trust. Everyone knows someone’s got their back, reducing the pressure to cut corners. Safety doesn’t just come from written protocols; it’s built from habits, shared experience, and honest talk about the mistakes that almost happened—or did.
Understanding Chloroacetyl Chloride
Chloroacetyl chloride shows up in a lot of industrial processes. The chemical formula stands as C2H2Cl2O. Some might list it out by its structure—something like ClCH2COCl—since it packs both an acetyl and a chloride group on a simple two-carbon backbone. It’s a clear, colorless liquid, sharp and biting in odor, and it reacts fiercely with water, so storing and handling it demands real care.
Why the Formula Matters in Real Life
Folks on the production floor rarely recite chemical formulas, but the formula points straight to risk. C2H2Cl2O means you have an acid chloride on your hands—think strong reactivity, a taste for moisture, and the potential to put chlorine gas into the air in the wrong conditions. That’s more than book knowledge. Anyone who’s ever had to evacuate a lab because of just a small spill will remember the panic and mess. No matter how much training or signage, complacency grows when you see the same clear liquid day after day.
Why Industry Uses Chloroacetyl Chloride
This isn’t a chemical that just sits on a shelf. Factories use it to make herbicides, certain antibiotics, and dyes. It’s a building block—one of those workhorses you find behind a ton of specialty products. The steps where it shows up make other chemicals possible—think phenoxyacetic herbicides, vital in agriculture, or pharmaceutical intermediates, the foundation for things like painkillers or infection-fighting drugs.
The Risks People Face
People working around chloroacetyl chloride face real dangers. It reacts quickly with water—so the air with a little humidity or even sweat can trigger a pretty rough reaction. Breathing in those fumes brings sore throats, coughing, and something a lot more serious with enough exposure. Skin contact causes burns that linger, and the eyes can take permanent damage. The chemical gives no second chances—just a reminder that safety equipment only works if used right every single time.
Responsible Management and Real Solutions
In every plant I visited, I saw the difference between places that talked safety and those where it actually mattered. The honest ones gave folks the gear, checked storage rooms for leaks, and provided real training. A decent ventilation system can make a world of difference. Routine monitoring turns guesswork into prevention. I learned fast: cutting corners meant calling in clean-up contractors and hoping nobody lands in the hospital.
Regulation matters, but daily habits decide the real safety record. Double checks on labeling, unambiguous instructions, and quick action drills turn chemical formulas into something workers respect instead of ignore. Digital tracking now lets managers review who accessed each storage drum and when, pushing personal accountability higher. It’s not just about ticking off a compliance box; real safety culture comes from leaders who care more about coworkers than quarterly reports.
Updates and Transparency
Public agencies keep pushing for better standards. Recent years saw stronger labeling laws, more required accident reporting, and campaigns to share best practices. Communities want transparency. Chemists, managers, and first responders know that hiding risks or skimping on safety doesn’t help anyone. The hope: a generation of workers who see C2H2Cl2O not just as a formula but as a responsibility.
Understanding the Risks
Chloroacetyl chloride holds an important spot in chemical manufacturing, but it packs serious hazards. Exposure can trigger violent reactions and release toxic fumes. I remember once walking through a lab where just a whiff called for evacuation. This chemical doesn’t forgive mistakes or shortcuts. Storing it safely isn’t about ticking off a checklist. It’s about people heading home healthy and communities kept safe from unnecessary leaks or explosions.
Key Factors in Storage
Routine is never enough here. Chloroacetyl chloride reacts fiercely with water, sending out clouds of hydrochloric acid and heat. Even small spills threaten both workers and the environment. Storage calls for dry, cool places. Only keep it in tightly sealed containers, using glass or certain coated metals—never anything reactive like aluminum or simple steel, which invite corrosion.
Ventilation makes a real difference. Many old storage rooms still rely on minimal airflow, but with chemicals like this, those days are over. Strong extraction systems let any stray fumes out, so they don’t build up to dangerous levels. Even during an earthquake or fire, air control systems need enough battery backup to keep running until the area clears.
Planning for Emergencies
No one stays comfortable with chemicals stacked up right outside the emergency exit. Facilities should put barrier zones around storage rooms. If a drum leaks or a bottle shatters, fire-retardant walls and chemical traps help keep a local problem from growing. Trained staff must know the drill for dealing with leaks or fires—no guessing, no improvisation. That means regular drills and the right gear available at every shift.
Spill kits get too little attention sometimes. Once, I saw a technician run from a spill site searching for supplies—no time for that. Spill control materials should always sit right where storage happens: neutralizing powders, gas masks, protective aprons, and eyewash stations with fresh water.
Respecting Transport and Documentation
Transporting chloroacetyl chloride opens the door to new risks. Secure containers, clear hazard labels, and standardized procedures all cut down chances for a mistake. Drivers trained in handling chemicals know what can go wrong fast. Each drum or bottle must come with well-kept documentation, including batch numbers and expiration dates, so tracking issues back to the source never turns into a guessing game.
Using Technology and Regular Checks
Smart sensors can flag any leaks before a human nose can notice them. Routine inspections spot corrosion, faulty seals, or missing labels. As regulations change, updates in protocols ensure old habits don’t lead to new disasters. Companies committed to ongoing training help keep both new hires and seasoned hands sharp.
Building a Culture of Responsibility
Accidents happen when storage gets sloppy or shortcuts become routine. Regular reviews, whistleblower protections, and open communication raise the odds that if someone sees a problem, they can speak up and get it fixed. As someone who has walked factory floors, I’ve learned you can spot the difference between places that just store chemicals and places where people feel responsible for safety. Those places see fewer surprises, healthier teams, and more trust from the community. Chloroacetyl chloride deserves no less.
What Chloroacetyl Chloride Can Do to the Human Body
One thing sticks out about chloroacetyl chloride: it punishes shortcuts in the lab or factory. This chemical burns skin in seconds. Once the vapors float into the air, eyes sting, throats tighten, and lungs can seize. I’ve seen workers in chemical plants, even those used to tough substances, move with extra care around containers labeled with its name. Chloroacetyl chloride reacts with water, even humidity in the air, releasing hydrogen chloride gas—a fume that bites deep into the airways.
Short-term exposure brings urgent trouble. Touching the liquid produces instant blisters. Breathing even low concentrations leads to coughing, shortness of breath, chest pain, and a choking sensation. The U.S. National Institute for Occupational Safety and Health (NIOSH) lists this compound as immediately dangerous to life and health at just 2 ppm. Higher concentrations, even for a few moments, may cause swelling in the throat or lungs, which can block airways entirely.
The crisis doesn’t end fast. Chloroacetyl chloride can seep into clothing and keep burning long after initial contact. If it splashes in the eyes, vision may never recover fully, even after prompt flushing. The risk isn’t just immediate damage; repeated or longer-term contact reportedly increases asthma-like symptoms and scarring in the lungs.
Impacts Beyond the Factory Floor
Spills don’t stop at the worker’s skin. Water and soil near release sites turn acidic fast, which harms aquatic life and plants. My own experience with small-scale cleanups taught me that even a minor mishap means full hazmat gear, fans blowing the fumes away, and a specialized cleanup crew. Communities living near chemical plants face the fear of leaks. Over the past decade, accidental releases have led to evacuation orders and long-term distrust of industrial neighbors.
In transportation, chloroacetyl chloride ranks among the chemicals that make emergency responders take the long way around. Leaks during shipping, which sometimes hit the news, force highways shut and rivers tested for contamination. The Environmental Protection Agency keeps close tabs on sites that produce or store this compound, proof that the danger hardly ends when production stops for the day.
Safer Pathways: What Works on the Ground
Real protection begins long before accidents. In every lab or plant, simple habits matter—double-checking labels, using protective gear, and limiting the number of people who handle the chemical at any moment. I’ve noticed that plants with regular drills and honest conversations about incidents often dodge the worst problems. The American Chemical Society suggests using containment cabinets and full-face respirators for a reason: shortcuts almost always backfire with compounds this harsh.
Industry can reduce risks by improving controls that detect and stop leaks instantly. Sensors that “smell” chloroacetyl chloride before people do now exist, along with remote shutoff valves. For transport, only trained carriers with reinforced drums make sense. Prompt medical care—oxygen, washing with copious water, immediate decontamination—saves lives, but the real trick lies in prevention.
Out in the community, people need honest, up-to-date information. Fact sheets, emergency phone lines, and routine drills build trust, not just compliance. Some of the best-run chemical zones I’ve seen rely on regular public meetings. Solutions start with openness and a willingness to invest in safety over speed.
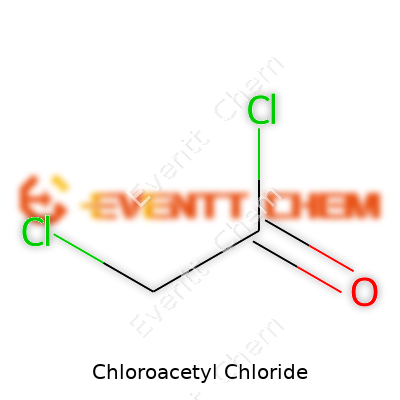

| Names | |
| Preferred IUPAC name | 2-chloroacetyl chloride |
| Other names |
CAC
Chloroacetylchloride Chloroacetic acid chloride Monochloroacetyl chloride 2-Chloroacetyl chloride |
| Pronunciation | /klɔːr.oʊ.əˈsiː.tɪl ˈklɔːraɪd/ |
| Identifiers | |
| CAS Number | 79-04-9 |
| Beilstein Reference | 1209224 |
| ChEBI | CHEBI:35983 |
| ChEMBL | CHEMBL135444 |
| ChemSpider | 13141 |
| DrugBank | DB14004 |
| ECHA InfoCard | 100.004.734 |
| EC Number | 204-614-3 |
| Gmelin Reference | 6377 |
| KEGG | C01834 |
| MeSH | D002687 |
| PubChem CID | 6047 |
| RTECS number | AF9925000 |
| UNII | PII4Z76Y8X |
| UN number | UN1752 |
| Properties | |
| Chemical formula | C2H2Cl2O |
| Molar mass | 112.94 g/mol |
| Appearance | Colorless to slightly yellow liquid |
| Odor | Pungent, suffocating |
| Density | 1.418 g/cm³ |
| Solubility in water | Reacts |
| log P | 1.77 |
| Vapor pressure | 24 mmHg (20°C) |
| Acidity (pKa) | 1.0 |
| Magnetic susceptibility (χ) | -7.4 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.462 |
| Viscosity | 1.17 mPa·s (20 °C) |
| Dipole moment | 1.90 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -259.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -422.7 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Toxic if swallowed, causes severe skin burns and eye damage, may cause respiratory irritation, reacts violently with water. |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS02,GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. H314: Causes severe skin burns and eye damage. H335: May cause respiratory irritation. |
| Precautionary statements | P210, P234, P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 3 3 1 Water-Reactive |
| Flash point | 75 °F (24 °C) - closed cup |
| Autoignition temperature | > 250°C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 oral rat 476 mg/kg |
| LD50 (median dose) | LD50 (median dose): 830 mg/kg (rat, oral) |
| NIOSH | GMG36950 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Chloroacetyl Chloride: 0.05 ppm (0.25 mg/m³) |
| REL (Recommended) | 0.05 ppm |
| IDLH (Immediate danger) | 1 ppm |
| Related compounds | |
| Related compounds |
Acetyl chloride
Chloroacetic acid Chloroacetyl-CoA Chloroacetamide Chloroacetonitrile Bromoacetyl chloride Dichloroacetyl chloride |



