Calcium Acetate Monohydrate: More Than Just a Chemical
Historical Development
People might not realize it, but calcium acetate has roots deep in history. The path really picked up steam in the early 19th century, when chemists started isolating and cataloging new compounds. As early as the 1800s, researchers studied calcium salts as minor curiosities. Pharmacopeias dating back almost two centuries mention this salt, but it barely left the laboratory bench back then. The real change started with the need to manage phosphorus in chronic kidney disease. By the late 20th century, companies began to make highly pure calcium acetate monohydrate for medicine, food, and specialty uses. This shift tied the story of the compound to human health and technology in a way that basic chemical texts could never predict.
Product Overview
A lot of folks mix up calcium acetate monohydrate with the anhydrous form, but the distinction matters. The monohydrate version always carries a water molecule with every formula unit. You see bags marked “pharma grade,” “food grade,” and “industrial.” Each serves specific needs. Medical suppliers use tight quality controls due to strict regulatory environments. Food producers hunt for batch-to-batch consistency. In the classroom, chemistry teachers count on a powder that dissolves quickly and reacts in predictable ways. Companies tend to ship it as a white, free-flowing powder or sometimes as small granules, in sealed plastic drums or paper sacks lined with polyethylene for moisture protection.
Physical & Chemical Properties
This salt never shines with the glamour of rare earth metals, but its performance depends on the details. Calcium acetate monohydrate appears as a white or nearly white crystalline powder. It smells faintly vinegary, not unpleasant, hinting at acetic acid. The real selling point comes from its solubility—in warm water or acidic conditions, it dissolves easily, unlike some calcium salts. It melts at a relatively low temperature and starts decomposing before boiling, releasing vapors that smell like vinegar. Each molecule binds tightly to a single water molecule, which matters in pharmaceutical handling. The monohydrate form isn’t just a technicality; it shifts the compound’s mass by a quantifiable amount, which has to be considered in laboratory work or medical dosing.
Technical Specifications & Labeling
Regulators in the United States and Europe insist on precise specifications. The United States Pharmacopeia sets a minimum threshold for calcium content—an amount that translates to accurate phosphate binding in the human gut. Particle size, heaviness (bulk density), moisture content, and heavy metal limits must meet strict caps, and the packing must clearly state “for food use” or “for pharmaceutical compounding.” The current global standard often asks for purity above 98 percent, with limits on lead, arsenic, and other metals set far below toxic thresholds. Handling labels call out its status as a non-hazardous material under normal use, though dust can cause minor irritation. Keeping it in a dry place becomes essential, as the monohydrate will readily absorb more water and clump if left exposed.
Preparation Method
Making calcium acetate monohydrate doesn’t require highly advanced technology but does call for tight control over raw materials. Most manufacturers begin with either calcium carbonate sourced from limestone or, less commonly, calcium hydroxide. Vinegar or glacial acetic acid provides the acetate side. By adding acetic acid to calcium carbonate, a bubbling reaction releases carbon dioxide gas, leaving calcium acetate dissolved in the mix. The liquid gets filtered to remove impurities, then crystallized at a controlled rate to form the monohydrate. This crystallization step demands careful attention to cooling rate, agitation, and purity of reagents; cut corners here, and the finished product may fail chemical assays. Post-crystallization drying must avoid overheating, or the water of hydration will escape, shifting the balance toward the less useful anhydrous form.
Chemical Reactions & Modifications
This compound reacts quietly but consistently. Drop it in water, and you get a mildly basic solution, quite useful for titrations or adjusting pH. Mix it with potassium oxalate, and you’ll see a white precipitate of calcium oxalate form—nothing to scoff at, since this reaction mirrors what happens with kidney stones. Chemists often turn to calcium acetate when preparing other calcium salts. Heat it strongly, and it decomposes, producing gases like acetone and acetic acid, which can ignite in air. In some specialty labs, researchers tweak its crystal lattice, swap parts of the acetate, or substitute heavy water to study subtle differences in reactivity and metabolism.
Synonyms & Product Names
Scan a chemical catalog, and the names jump out: “Calcium diacetate monohydrate”, “E263”, or just “CalAc.” Pharmaceutical compendia call it “Calcii Acetas Monohydricus” for Latin flavor. In China, bags might read “乙酸钙一水合物.” Each name reflects an attempt to fit this material to an application. On food labels, “E263” signals a permitted preservative. Companies sometimes market it as “phosphate binder” for dialysis clinics or as “acidity regulator” in snack foods. This shifting parade of names keeps the compound’s identity both global and local.
Safety & Operational Standards
Experience teaches that minor mishandling rarely causes trouble with calcium acetate monohydrate. Still, complacency carries risk. Inhaled dust can irritate the nose and throat, not a crisis, but enough to matter for a worker handling big sacks all day. Wet floors get slippery. No one has yet linked this compound to chronic toxicity at the levels people encounter in pharmacies or food plants. Still, processors wear dust masks and gloves, keep the powder off skin, and replace spilled product promptly. Safety data sheets warn against mixing it blindly with strong oxidizers or acids, though such reactions rarely occur outside of a lab setting. Regulatory bodies ask for traceability back to batch origins, echoing lessons learned from food scandals and drug recalls. Fire crews treat larger spills as low concern, since it does not burn and releases non-toxic byproducts.
Application Area
Modern medicine depends on reliable phosphate binders, and calcium acetate now stands as a front-line option, particularly for patients on dialysis. Doctors prescribe it to control blood phosphorus when kidneys fail to do the job. Food manufacturers use it under the code E263, mainly to avoid spoilage in baked goods and processed snacks, because it slows down mold growth. The construction industry taps into its ability to set cement and plaster faster, especially in cold weather. Laboratory chemists use it as a calcium ion source, preferring its quick dissolve over the sluggishness of other salts. A cheese maker might rely on it to control acidity during ripening, while environmental remediators add it to waste streams when they need predictable chemical reactions and easy cleanup.
Research & Development
Research on calcium acetate monohydrate does not run dry. Pharmaceutical scientists probe new delivery forms for patients with kidney failure—chewable tablets, slow-release beads, and even flavored powders for easier use in children. Food technologists tune its use to curb microbes without altering taste or texture in breads and cakes. Animal feed researchers dig deeper into its effect on phosphate absorption in livestock, hoping to balance animal nutrition with environmental sustainability. Analytical chemists develop better ways to detect trace levels in food and medicine, chasing ever-lower contamination thresholds. In the past five years, work on greener synthesis tracks the shift away from fossil-derived acetic acid toward more renewable, bio-based feedstocks. Each new journal publication pushes this compound a little farther, sharpening its use and safety.
Toxicity Research
The safety record looks reassuring, but modern toxicology never lets up. Animal studies indicate a wide margin between functional doses and toxic effects. Calcium acetate breaks down to calcium and acetate in the gut, both well-known nutrients. Problems seem to come only with large, repeated doses, which can tip the body’s calcium balance out of whack, causing constipation or, rarely, kidney stones. The most serious risk shows up in patients with advanced kidney disease, who can’t excrete extra calcium and might wind up with dangerous deposits in veins or organs. Over the decades, post-market surveillance spotted only a handful of adverse events, almost always tied to medical overdosing or long-term use beyond the intended population. Regulators lean on this evidence but keep pushing for more thorough research, especially with the trend toward adding more calcium to more foods.
Future Prospects
Much as it sounds like a solved problem, the future of calcium acetate monohydrate twists with changing needs. The steady rise in chronic kidney disease—driven by diabetes and aging—will keep cementing its place in healthcare worldwide. Meanwhile, food industries test new uses to block spoilage with lower additive levels, in line with consumer pushback against “chemicals” on labels. Clean-label advocates lean into its “naturally occurring” status, but business depends on tight proof of origin and safety. Research labs probe calcium acetate’s role in supplement blends and as a delivery agent for micronutrients in malnourished populations. Some environmental engineers float ideas for using it in water purification and soil stabilization, hinting at applications still waiting on the right commercial push. It sits at the intersection of medicine, food, and industry, where simplicity counts as much as innovation. Each new study peels back more about its strengths—and its limits.
Calcium Acetate Monohydrate: More Than a Simple Compound
Calcium acetate monohydrate sounds like something you only come across in a laboratory, though most people have interacted with it without even realizing. Pharmacies, food processing plants, wastewater treatment facilities—this chemical pops up all over the place, quietly working behind the scenes in ways that affect more lives than you might expect.
Keeping Kidneys Happy: Medical Uses
One of the clearest examples of its importance shows up in dialysis clinics. People with chronic kidney disease need to keep phosphate levels in check. High phosphate can damage bones, blood vessels, heart, and skin because their kidneys can’t filter it out anymore. Doctors turn to calcium acetate monohydrate because it helps trap the excess phosphate from food in the digestive tract. This way, less of it ends up in the blood. As someone who has walked a loved one through dialysis, seeing phosphate levels drop from dangerously high to steady brings a sigh of relief each time the routine lab results come in.
Food Preservation and Safety
Some might spot calcium acetate on a food label and not think twice. But as a food additive, it keeps baked goods stable and acts as a preservative. Bakers value the way it helps prevent spoilage and mold growth. Without food-grade compounds like this, keeping bread safe and edible for several days would be a real struggle. Food safety agencies keep a close eye on how much goes into processed food, and evidence shows typical use doesn’t create health concerns for the general population.
Water and the Role of Cleaner Environments
Water treatment plants face plenty of challenges, especially as our communities keep growing. Phosphorus pollution, mostly from fertilizers and detergents, clogs up lakes and rivers, triggering harmful algae blooms. Calcium acetate monohydrate helps bind up this phosphorus so it can be pulled out before treated water flows back into the environment. Anyone who loves fishing, swimming, or just watching nature bounce back after cleanup measures can appreciate what this chemical offers to water engineers.
Industry and Everyday Life
Industrial operations use calcium acetate in surprising spots. Chemical manufacturers mix it into processes that need a reliable buffering agent. The plastics industry uses it to stabilize certain products. Firefighters carry it in some types of fire extinguishers, since it can help knock down certain kinds of flames. Growing up in a family of firefighters, I picked up more than a few stories about odd-seeming chemical tools packed in their trucks, and calcium acetate monohydrate had a spot on that long list.
Safety, Misinformation, and Smarter Choices
Whenever a chemical with so many applications comes up, worry isn’t far behind. Some online posts lump all additives together and urge people to keep anything unpronounceable out of their diets or routines. Experience—and a healthy dose of research—teaches that risk depends on the dose, the way something is used, and the trustworthiness of those handling it. Health authorities in the US, EU, and other regions set clear safety benchmarks. Sticking to known sources and reading up before panicking makes a difference. For anyone feeling overwhelmed, healthcare providers can explain any personal risks, especially for those with calcium or kidney conditions.
Small Compound, Broad Impact
Instead of brushing past the fine print or wringing hands over chemical names, start with some honest questions. Calcium acetate monohydrate keeps foods safe, supports people with serious illness, and helps protect water supplies. A little chemistry, checked by sound evidence, does plenty of good behind closed doors—and on the kitchen table, too.
Understanding Calcium Acetate Monohydrate
Calcium Acetate Monohydrate shows up in many medicine cabinets, mostly for its role in controlling blood phosphate levels, especially for patients dealing with kidney disease. Doctors use it to keep the phosphorus in check, which helps slow down or prevent bone problems and other complications that come up when the kidneys falter. You find it prescribed for people on dialysis, not so much for others.
So, What's the Recommended Dose?
Doctors usually suggest starting at 1,334 mg of Calcium Acetate Monohydrate, taken with each meal. That's three times a day. The compound binds dietary phosphorus in the gut, meaning it doesn't get sucked into your bloodstream. Over time, doctors adjust the dose, usually every 2-3 weeks, based on regular blood phosphate testing.
Most folks need between 2,668 mg and 8,002 mg per day, split across meals. Any more, and you might start running into issues like high calcium in the blood, stomach pain, or constipation. That’s why this drug always stays a prescription-only option. Trying to tweak doses on your own can land you in deep trouble.
Why Proper Dosing Matters
If you've ever watched someone facing kidney failure, you notice that phosphate builds up fast. It doesn't just stick to bones; it sneaks into arteries, skin, and tissues, bringing pain, itching, and heart troubles. Some people think, “More is better,” but with calcium-based binders, that can flip your blood calcium higher than needed, setting the stage for dangerous heart rhythms or even kidney stones.
The danger is real. Studies from the National Kidney Foundation hammer home that dosing too high or too low increases the risk for long-term complications. Staying in the sweet spot between 2.5 and 4.5 mg/dL for blood phosphate keeps bones and blood vessels in better shape. Guidelines recommend close monitoring by a medical team for exactly this reason. I remember a patient who thought doubling his dose after a “heavy meal” would help. He ended up in the ER, fighting severe constipation and calcium levels well above safe limits.
How To Get Dosing Right
Doctors stress regular blood tests. They don’t just measure phosphorus; they check calcium levels too, along with kidney function steps like parathyroid hormone. Without this, even the best intentions can go sideways. If a meal has more phosphorus—think dairy, nuts, cola—a patient might need a higher dose, but only if the doctor says so. Otherwise, the safest move is to stick to the plan and keep up with appointments.
Better Solutions on the Horizon
The world of phosphate binders keeps moving. Newer non-calcium options, like sevelamer and lanthanum, offer other paths for folks who can't tolerate higher calcium. These alternatives lower phosphate without bumping up calcium levels. Of course, cost and insurance impact availability, so not everyone gets access right away. Doctors and patients talk often to balance risks and benefits, sometimes switching up drugs based on lab results or side effects.
The take-home message: Any adjustment to calcium acetate monohydrate—up or down—should happen with a clear plan from a trusted professional. This isn’t a supplement you pick up at the store and try on your own. Good health grows with teamwork, steady monitoring, and honest back-and-forth with your care team.
What Happens After Taking Calcium Acetate Monohydrate
Calcium acetate monohydrate finds a place on plenty of pharmacy shelves because it can help manage phosphate levels in people with kidney problems. Doctors often reach for this compound when patients struggle with chronic kidney disease. Bringing phosphate down protects bones and blood vessels. Yet, like anything that changes the body’s chemistry, this medicine does not come without risks.
The Most Common Side Effects
Digestive issues top the list. After taking calcium acetate monohydrate, people sometimes run into problems like constipation, upset stomach, or even nausea. Sometimes, bloating and gas creep in. Most people can relate—a shift in diet or new supplement tends to shake up digestive patterns. This drug binds with phosphate in the stomach and intestines, so it makes sense the belly feels it most.
Another common complaint comes from that same digestive irritation: vomiting and abdominal pain. If someone has dealt with irritable bowels or struggled with harsh-tasting medicines before, the discomfort may feel familiar. These symptoms usually subside once the body gets used to the compound or if a doctor tweaks the dose.
What About Calcium Buildup?
The bigger concern centers around calcium itself. If a person takes too much, there’s a real chance for calcium levels to climb too high in the blood—a condition called hypercalcemia. This can make someone feel tired, thirsty, or cause them to urinate more often. It takes a toll on the kidneys, heart, and sometimes creates confusion or muscle weakness. Doctors usually spot this risk with routine blood tests and keep a close watch on symptoms.
Who Faces Extra Risk
Some people brush off mild side effects, but for those with certain health backgrounds, even a small problem can snowball. Patients using digitalis for the heart need careful observation, because calcium can worsen toxicity and trigger dangerous rhythms. Anyone with a history of kidney stones should bring this up with their doctor, since extra calcium can make stones more likely. Seniors, and those already struggling with too much calcium, must tread carefully.
Behind the Scenes: Balancing Benefits and Risks
Balancing the benefits of lowering phosphate against these side effects requires teamwork between patients and healthcare providers. Blood work, communication, and a willingness to call the doctor if something feels off—these all play a role.
Having used phosphate binders in my own family, I’ve seen how real-world side effects can shape daily life. A small tweak in meals or splitting up doses sometimes solves the problem. Hydration also plays a big part. Drinking enough water can keep constipation and kidney strain under control.
What Can Help? Practical Tips
Simple steps can make a big difference. Patients should take the medicine with food, as prescribed. Writing down symptoms or changes in mood, appetite, or bathroom habits gives the doctor useful clues. Bringing in a record to the next appointment saves time and trouble. Lab checks line up with this approach, showing if calcium or phosphate swings out of range.
Education from a doctor or pharmacist goes a long way. Knowing what warning signs to watch for helps catch problems early. Seeking medical advice promptly—especially with symptoms like confusion, irregular heartbeat, or severe stomach pain—can prevent an issue from worsening.
Using calcium acetate monohydrate as part of kidney care takes vigilance. With good habits, honest conversations, and a bit of patience, side effects can be kept in check and the real benefit of the treatment reached.
The Role of Calcium Acetate Monohydrate
Phosphorus control often becomes a daily battle for people living with kidney disease, especially those on dialysis. High phosphorus in the blood leads to bone problems, heart disease, and a whole list of long-term complications. Doctors have leaned on phosphate binders like calcium acetate monohydrate for many years because it traps phosphorus in the stomach and stops it from being absorbed.
If you’ve ever been in the kidney clinic or spent a day with someone going through dialysis, you’ll notice that food choices and medicines form the rhythm of daily life. Calcium acetate isn't an exotic medicine. It’s a white powder, pressed into tablets or capsules, designed to handle the job with meals. Compared to alternatives like aluminum-based binders, it comes with fewer horror stories about bone disease or mental cloudiness. Still, no medicine gets a free pass, and patients and doctors spend a lot of time weighing side effects, long-term results, and lab numbers.
Safety Concerns and Real-Life Experience
So, is calcium acetate monohydrate really safe for those with kidney problems? It’s safer than some older binders, but not perfect. Studies point out one concern repeatedly: hypercalcemia, or high calcium levels in the blood, creeps up if too much calcium acetate is taken. This problem leads to its own set of headaches—calcification of blood vessels, a higher risk of heart disease, and not-so-great outcomes for people already struggling with kidney trouble. The National Kidney Foundation, along with other major guidelines, push for regular calcium level checks any time calcium acetate is used.
Most people on dialysis also take active forms of vitamin D, which boost calcium absorption. This makes it easier for their calcium levels to tip too high. I’ve seen patients who felt fine one month and started feeling tired or having strange muscle twitches the next. Their labs told the story: calcium edging upwards, sometimes alarming enough to stop the medication altogether.
Constipation and upset stomachs also pop up a lot with calcium acetate. One of my relatives on dialysis used to say he could handle the kidney machine, but not the battles with stubborn bowels from all the binders and iron pills. These “minor” annoyances can really slow down someone’s day and ding their quality of life.
Balancing Risks and Better Options
Doctors try to keep total daily calcium (diet and binders together) under recommended limits, usually around 2,000 milligrams a day. This means regular talks about food—milk, cheese, anything fortified. Many clinics run monthly blood checks, and personal experience says those numbers end up shaping decisions more than the best guidelines.
There are now non-calcium binders like sevelamer and lanthanum. They sidestep the calcium overload but tend to hit the wallet harder and sometimes come with their own awkward side effects. Calcium acetate still holds a place in many treatment plans because it’s accessible, effective, and familiar in most clinics.
A solid solution relies on a team effort—doctors, dietitians, patients, and family. No one-size-fits-all trick exists, so talking openly about side effects and watching lab numbers remains the best way to keep calcium acetate monohydrate safe. For some, switching to a non-calcium binder works better, while for others, careful use with plenty of monitoring keeps complications in check. Access to affordable choices helps as well, so no one feels stuck with only one option for managing their health.
More Than a White Powder
Calcium acetate monohydrate comes up a lot in pharmacy shelves and food labs. Anyone who has handled it knows this compound isn’t just “another chemical” to toss on a shelf. Its well-being, and our safety, depend on how we treat it after delivery. Lax storage turns useful materials into headaches.
Humidity Spoils the Game
I spent a few years managing the supplies room in a midsize hospital pharmacy. Humidity wreaked havoc, plain and simple. Calcium acetate monohydrate draws water from the air, sticking together into useless clumps. Once this happens, measuring out an accurate dose or even scooping a clean sample gets tricky. Data from manufacturers back this experience: they recommend a cool, dry storage spot at all times. If you find powder starting to cake or clump, it’s more than an inconvenience; it risks inaccuracy in medicine, in food, and in research.
Temperature and Its Role
Nobody likes extremes. That goes for calcium acetate monohydrate, too. At room temperature, it remains stable. I’ve seen what warm storerooms do—degradation speeds up. Chemical breakdown not only ruins the substance, but also poses risks to those who use the product. The World Health Organization highlights room temperature (15-25°C) and dryness as the gold standard for pharmaceuticals. Keep it away from heat, radiators, sun-drenched counters, and high-powered lights.
Why Sealed Containers Aren’t Optional
Working in busy labs, I’ve noticed how cracked lids and loosely wrapped jars might look “good enough.” They aren’t. Dust, pests, and especially moisture sneak in, accelerating spoilage. Air-tight, clearly labeled containers with easy-to-read dates keep everything in order. If a spill happens, grab gloves, sweep up, and follow disposal instructions. This isn’t about red tape, it’s self-respect and protecting coworkers.
Chemical Compatibility: Some Roommates Don’t Mix
Calcium acetate monohydrate plays poorly with acids and strong oxidizers. I once saw a storage mishap where someone parked a jug next to a leaky bottle of sulfuric acid. Neutralization or worse could happen fast. Every chemical shelf should group similar stabilities and incompatibilities together. A color-coded system or detailed inventory prevents minor mistakes from growing teeth.
Labeling and Rotation—Small Steps, Big Gains
Every container should wear a clear label: name, date received, expiration date. I learned early that rotation matters; new shipments behind old stock. If a batch sits long past its use-by-date, don’t “just use it”; it’s not worth the risk. Most professional organizations and food producers drive this point home, and quietly tossing out questionable product has saved more than one batch of pills or food from being ruined.
Training and Good Habits Make the Difference
Fancy climate controls can only do so much. Training staff to check lids, watch for odd smells or textures, keep proper logs, and react quickly to spills beats fancy equipment every time. Even in small operations, taking ten minutes to review safe storage keeps everyone out of trouble. Regular checks, a good thermometer and hygrometer, and clean working spaces—these simple moves prevent most issues.
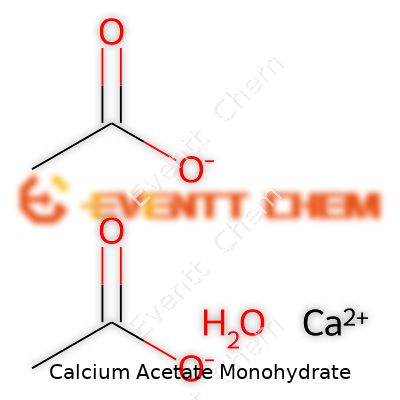
| Names | |
| Preferred IUPAC name | Calcium diacetate monohydrate |
| Other names |
Calcium ethanoate monohydrate
Acetic acid, calcium salt, monohydrate |
| Pronunciation | /ˈkæl.si.əm ˈæs.ɪ.teɪt ˌmɒn.oʊˈhaɪ.dreɪt/ |
| Identifiers | |
| CAS Number | [5743-26-0] |
| Beilstein Reference | 7784437 |
| ChEBI | CHEBI:86156 |
| ChEMBL | CHEMBL1201777 |
| ChemSpider | 86434 |
| DrugBank | DB11092 |
| ECHA InfoCard | 03f00b59-7a4a-4516-8ab1-9d70acda9d56 |
| EC Number | 208-140-2 |
| Gmelin Reference | 62612 |
| KEGG | C02436 |
| MeSH | D018437 |
| PubChem CID | 173137 |
| RTECS number | AF7385000 |
| UNII | GX81Q0S9T5 |
| UN number | UN1841 |
| Properties | |
| Chemical formula | Ca(C2H3O2)2·H2O |
| Molar mass | 176.18 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.0 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.4 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 12.6 |
| Basicity (pKb) | 9.3 |
| Magnetic susceptibility (χ) | -4.1 × 10⁻⁶ cm³/mol |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1627.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1776 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A12AA04 |
| Hazards | |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H319 Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 oral rat 4,290 mg/kg |
| LD50 (median dose) | LD50 (Oral, Rat): 2,600 mg/kg |
| NIOSH | N0947 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 1200 mg/kg bw |
| Related compounds | |
| Related compounds |
Calcium acetate
Calcium carbonate Calcium chloride Magnesium acetate Sodium acetate Calcium lactate |



