Butyl Acetate: Insight into an Ubiquitous Solvent
Historical Development
Interest in butyl acetate stretches back over a century, as chemists began to recognize the unique combination of volatility, pleasant odor, and solvent power this ester offered. Traditional organic synthesis relied on natural sources or cumbersome processes, but the industrial age brought forward reliable methods for large-scale production. In the paint and coating boom of the 20th century, butyl acetate found favor for its ability to dissolve cellulose nitrate, and whether the end users realized it or not, this solvent helped transform manufacturing lines and enabled faster drying times for wood finishes, car paints, and inks. This evolution encouraged more focused research into derivative compounds and safer handling, bringing us to today’s tightly regulated, high-purity production environment.
Product Overview
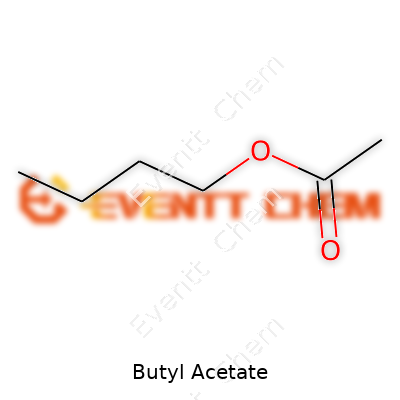
Butyl acetate, or n-butyl ethanoate, is made by a straightforward reaction between butanol and acetic acid, often under acidic catalysis. This clear, colorless liquid stands out thanks to a sweet, fruity odor reminiscent of apples or bananas. Commercially, it appears in varying grades, from technical to analytical purity, packaged in drums or bulk for industry use. The appeal comes from a tangible combination of performance and affordability, offering an evaporation rate just right for spray lacquers or ink jet formulations. Chemical suppliers use terms like “normal butyl acetate,” “n-butyl acetate,” and “acetic acid butyl ester,” so keeping an eye on trade names matters for procurement teams.
Physical & Chemical Properties
A volatile, flammable substance, butyl acetate features a boiling point around 126°C, density near 0.88 g/cm³, and solubility in water topping out at roughly 0.7g per 100 mL at room temperature. It mixes well with ethanol, ether, and most common organic solvents. These traits appeal to chemists who need efficient blending during manufacturing. Its refractive index measures about 1.394; the flash point sits around 22°C, putting it well within the range for flammable liquid classification. The ester group, prone to hydrolysis, gives it some vulnerability in moist environments, but the volatility and pleasant aroma turn this to advantage in consumer applications like nail polish or flavorings.
Technical Specifications & Labeling
Product quality often gets determined by purity, water content, acidity (usually measured as acetic acid), and non-volatile matter. Technical sheets regularly specify minimum content above 99%, residual water lower than 0.1%, and acid value less than 0.01%. Storage requires proper labeling under international transport guidelines. With the UN number 1123, it calls for flammable liquid stickers and adherence to strict stack height in warehouses. European systems list it under EC 204-658-1, reinforcing the importance of traceability all along the supply chain. Workers handling it in process plants see MSDS documents outlining both hazard and environmental risks, while end users rarely consider such details, drawn in by performance rather than paperwork.
Preparation Method
Most plants prepare butyl acetate by the Fischer esterification process, pushing butanol and acetic acid together under sulfuric acid catalysis. Efficient separation and distillation follow, driven by the need to meet purity parameters. As with many esters, removing water as the reaction proceeds tips the scale toward product formation; this often involves azeotropic distillation. Companies focused on green chemistry sometimes turn to solid acid catalysts or enzyme systems for selectivity improvement, though cost and scaling issues limit widespread adoption. Reactant purity and catalyst longevity both matter; impurities can cloud the final liquid or speed up equipment wear, pushing up operational costs and quality control headaches.
Chemical Reactions & Modifications
Reactors filled with butyl acetate could see hydrolysis if water sneaks in, breaking the molecule back into butanol and acetic acid. Exposure to strong bases or acids speeds up that reverse route, so plant operators watch pH closely. In organic synthesis labs, butyl acetate sometimes acts as a starting point for more complex esters. It also sometimes gets used as a phase transfer agent, bridging polar and non-polar systems. On occasion, attempts at chemical modification target the butyl group itself, setting the stage for new esters or etherifications. Consistent behavior through most organic transformations helps keep costs down and planning straightforward.
Synonyms & Product Names
Different industries know butyl acetate by names like n-butyl ethanoate, butyl ester of acetic acid, or by CAS Number 123-86-4. In Asian markets, it sometimes appears under specific manufacturer names or shorter trade codes. Paint and coatings suppliers often list it as “normal butyl acetate,” to distinguish from isobutyl or tert-butyl acetate cousins, each with slightly altered properties. Regulatory documents keep track of all synonyms to avoid confusion across global operations, because a shipping mistake could mean bigger safety or compliance consequences. Whatever the label, the product inside carries that recognizable sweet, solvent smell.
Safety & Operational Standards
Butyl acetate brings clear hazards: high flammability, moderate toxicity on inhalation, and risk of irritation on prolonged exposure. Storage rules demand cool, ventilated spaces away from sparks or flame. Companies lean on local and international bodies for guidance, such as OSHA in the US or REACH in Europe. The workplace limit sits around 150 ppm for an 8-hour shift, reflecting its potential to cause headaches, dizziness, or eye and respiratory irritation. Fire safety systems, spill control kits, and personal protective equipment all sit within arm’s reach at busy production lines. Fire departments train for ester spills using foam extinguishers, not water. Environmental limits cap the amount of butyl acetate entering wastewater, so on-site scrubbers and separators often kick in before discharge.
Application Area
Walk into a car plant, print shop, or beauty salon, and butyl acetate is already in action. Automotive paints rely on this solvent to balance viscosity and drying, avoiding runs and sagging. Printing inks use it for quick-setting properties that allow for sharp, smudge-resistant graphics. In nail polish, it serves as the main carrier, giving products a smooth glide and that signature salon scent. Heavy industry uses butyl acetate as a degreaser, relying on its ability to cut through oil-based residues. Even in perfumery and food flavorings, trace amounts bring out sweet, fruity notes, although food-grade formulations go through extra purity testing. Companies appreciate how a small tweak in ratio or grade opens up application flexibility, right down to specialized coatings for electronics and wood furniture.
Research & Development
Scientists explore new catalysts that lower the temperature or boost the yield of esterification. Work continues in green chemistry labs, including bio-based routes starting from renewable feedstocks. Part of this research aims to reduce environmental impact and build circular economy pathways, letting used solvents return as feedstock for fresh batches. Analytical chemists refine detection and quantification methods to guarantee batch integrity. Engineers look for energy savings in distillation and separation, since processing accounts for a big slice of production cost. In universities and industrial pilot plants, work on safer alternatives and improved performance keeps butyl acetate at the center of solvent science discussions.
Toxicity Research
Acute exposure tests in animals have pointed to moderate toxicity, with main effects on the central nervous system, liver, and kidneys at high doses. Short-term inhalation exposure above threshold levels leads to headaches, drowsiness, and occasional eye, nose, or throat irritation. Chronic studies haven’t shown strong evidence of cancer or birth defects in humans at typical exposure levels, though research continues into possible links. Regulatory agencies keep updating recommended workplace air limits. Some researchers investigate safer handling practices and better early warning detection systems, since small leaks can quickly reach hazardous concentrations in confined settings. Concerns over environmental effects appear around aquatic toxicity and potential for bioaccumulation, even if low, pushing for better solvent management protocols and spill response readiness.
Future Prospects
Eco-friendly chemistry sits front and center for butyl acetate’s future. Bio-based routes promise reduced greenhouse emissions and better sustainability credentials, though scale-up and raw material prices remain hurdles. Advances in catalysts or membrane technologies might cut energy use and strengthen process economics. As industries look to phase out more toxic solvents, butyl acetate stands as a benchmark for performance and compliance with strict environmental or safety standards. Digital monitoring of solvent emissions, tighter waste controls, and improved labeling make compliance easier for both large plants and small businesses. Researchers working on solvent blends and coatings continue driving innovation, and given the expanding reach of paints, plastics, and formulated products, butyl acetate will likely keep its spot in the chemist’s toolkit—shaped by both regulatory push and marketplace pull.
Butyl Acetate in Paints and Coatings
Walk down any hardware store aisle, and there’s a good chance the paint cans lining the shelves contain butyl acetate. Manufacturers look for a solvent that improves how paint spreads and dries, and butyl acetate delivers on both fronts. It thins the mixture just right, keeps pigments from clumping, and leaves less chance for unsightly brush marks. In my own home, I’ve found paint jobs come out smoother when using products that lean on this solvent compared to cheaper alternatives. According to the European Chemicals Agency, solvent-based paints owe much of their ease of application to butyl acetate.
Found in Nail Polish and Cosmetics
A bottle of nail polish promises color and shine, but without a quick-drying agent like butyl acetate, putting on a fresh coat could become an hours-long ordeal. The beauty industry prizes butyl acetate because it evaporates at just the right speed, leaving color behind instead of a tacky mess. Some brands promote “no acetone” removers, assuming it's gentler, but butyl acetate’s mild scent and relatively low toxicity put it on the preferred list for both polish and remover.
Fragrances, Food Flavoring, and Everyday Smells
The whiff of green apples or pears can come from natural fruit, but food scientists also turn to butyl acetate for flavoring. The U.S. Food and Drug Administration classifies it as Generally Recognized as Safe (GRAS) for use in food flavorings, which says a lot about its low risk at levels found in products like gummy candies or fruit drinks. That fruity scent in perfume or body spray? It might use butyl acetate as a carrier—not only because it dissolves other scent ingredients, but also because of its own mild, pleasant aroma.
Role in Industrial Cleaning and Ink
Industrial cleaners often work fast and cover a lot of ground. Tools and machinery pick up grease that won’t budge with water alone, so cleaning solutions turn to butyl acetate for its ability to cut through oils and resins. Printers and press operators depend on it to keep ink flowing smoothly. In printing plants, this solvent helps ink stick evenly to paper, whether it’s a magazine or a product label. Without it, we would probably see more smears and less color pop.
Safety and Health Perspectives
Spending long days painting, I’ve learned not to ignore ventilation. Butyl acetate’s fumes can cause dizziness and headaches if the air gets stale. Occupational Safety and Health Administration (OSHA) reports that repeatedly breathing it at high concentrations does affect the nervous system, so workplaces put safety protocols in place to prevent overexposure. Storing cans and keeping doors open turns nuisance into minor detail. Knowing where it’s hiding, especially in household or DIY use, helps avoid accidental problems down the road.
Looking at the Big Picture
Butyl acetate makes modern living possible in ways rarely noticed. Demand for this chemical won’t disappear as long as we want smooth paint on walls, shiny nails, and good-smelling products. Transparency about ingredients, safe manufacturing, and informed choices matter. Companies who prioritize safety, workers who use protective gear, and people who read labels all play a role in keeping things safe. Sometimes it’s easy to forget the chemistry behind everyday life, but a little knowledge makes wiser consumers and a safer home.
Getting Real About Butyl Acetate
Butyl acetate shows up in plenty of places, from paint thinners to nail polish remover. Anyone working around it quickly learns that its sweet, fruity smell hides a set of risks. My days in a small print shop introduced me to the sharp headaches after fifteen minutes of carelessly handled solvent. Lessons like those stick, and they’ve shaped my view that certain habits matter more than warning labels ever can.
Why Ventilation Becomes a Game Changer
Good airflow needs to take priority any time butyl acetate comes out. Just cracking a window won’t cut it. Fume hoods or exhaust fans put a real dent in the vapor, so we don’t end up breathing in more than we realize. The American Conference of Governmental Industrial Hygienists sets limits for how much you can inhale before your health takes a hit. Chronic exposure can leave damage that turns up months later. Nothing beats a workspace designed for chemical handling with solid airflow pulling fumes away.
Personal Protective Equipment: Not Just for Show
Latex gloves don’t handle butyl acetate; those break down fast and let the solvent through. Nitrile or neoprene gloves actually stand up to the stuff. Eye protection becomes vital the minute there’s a splash risk. A plastic face shield or snug goggles can keep the pain of chemical burn out of your day. Fast access to eyewash stations brings peace of mind, even if you never have to use them.
Watching Out for Skin and Eye Contact
Even short exposure sets off a sharp burn, especially in any cuts or scratches. Skin absorbs solvents almost invisibly—damage can creep up without any dramatic symptoms. I met more than one co-worker who shrugged off a spill and missed developing dermatitis until it was a real problem. Shirts with long sleeves, pants that fit well, and covered shoes offer another barrier when gloves alone can’t keep up.
Handling Spills and Storage
Spills seem inevitable in busy workshops. Instead of panic, a simple routine gets everyone through it with less stress. Absorbent pads and proper disposal bins help make cleanup less dangerous. Drains need guarding, since butyl acetate causes bigger problems if it enters water systems.
Fire risk stays high around this solvent. It catches with a spark, and vapors hang close to the ground. Keeping fire extinguishers nearby—especially those rated for chemical fires—fends off disaster. Metal safety cans work better than open containers, and storing them in cool, well-marked cabinets cuts down the risk even more.
Training and Labeling Matter
No one feels like reading long labels in the middle of a busy shift. Still, labels and up-to-date training can mean the difference between a close call and a trip to the ER. Clear instructions posted near workstations, regular safety walk-throughs, and practice drills help people remember what needs doing when it counts the most. I’ve seen more accidents come from confusion or ignorance than genuine recklessness.
Building a Team Culture Around Safety
For all the technical advice, what really works is an attitude that safety is everyone’s job. People who watch out for each other catch mistakes a rulebook would miss. In my experience, sharing personal stories about risky moments changes minds faster than any formal training. Everyone learns faster when the stakes feel real. The smartest workplaces aim for zero incidents—not because it’s the rule, but because everyone wants to head home in good shape.
Butyl Acetate: In the Lab and on the Job
Walking through a paint factory or a nail salon, the sharp, sweet scent in the air points to a key ingredient. Butyl acetate is responsible for that familiar smell. Its chemical formula is C6H12O2, a simple combination of carbon, hydrogen, and oxygen. This clear liquid packs more punch than its formula suggests, turning up in day-to-day life far more often than most realize.
Why Butyl Acetate Catches Attention
Growing up with a father in the construction business, I spent summers surrounded by paint cans and solvents. Sometimes my nose would tingle from the fumes as my dad rolled walls or stripped varnish from old cabinets. Butyl acetate works as a powerful solvent, dissolving resins and plastics smoothly. That’s the science, but real-world jobs care about results. In a fast-paced workshop, time matters. Butyl acetate’s quick evaporation speeds up drying, keeps tools clean, and those clear advantages drive its popularity.
Not all formulas share this versatility. C6H12O2 stands out for use in automotive finishes, printing inks, synthetic flavors, and even as a fruit-flavored additive in candy and gum. Chemists recognize it for more than its smell—it allows flexibility in production and cuts costs for manufacturers. According to the European Chemicals Agency, tens of thousands of tons move through markets each year, driving a sizable economic footprint.
Concerns That Demand Respect
While its usefulness is clear, butyl acetate is not something to take lightly. The same characteristic that lets it strip paint also irritates eyes, nose, and skin. Even brief exposure in closed rooms may lead to headaches, dizziness, or sore throat—I’ve had days in college labs where a poor ventilation system meant all three. The U.S. National Institute for Occupational Safety and Health recommends regular fresh air breaks and high-quality respirators for anyone exposed for more than a few minutes. Stories about accidental overexposure remind workers how important it is to treat chemical safety seriously, especially with something as easy to underestimate as butyl acetate.
Companies have made big strides since my father’s painting days. Manufacturing guidelines now ask for locked storage, spill kits, and chemical-resistant gloves—even in small batches. Training workers to spot symptoms of overexposure keeps more people safe, and industry watchdogs push for labeling that makes hazards easy to spot right on the container. At the consumer level, better labeling on nail polish and craft paints helps parents and home hobbyists stay out of trouble.
Safe Use and Smarter Solutions
Solvents bring progress, but safety measures lag behind if taken for granted. Schools and workplaces with up-to-date engineering controls, good room ventilation, and routine air tests set the standard for health. Researchers at the American Chemistry Council have focused on improvements like less-harmful alternatives and closed-loop systems to limit fumes. Companies that invest in these improvements rarely regret it — they avoid costly legal cases and, more importantly, protect their workers.
Regulation pulls from the facts. C6H12O2 gives manufacturers a useful, affordable option, but ignoring safety for speed is no longer just risky; it’s unnecessary. Every person who reads a label, opens a window, or puts on gloves helps drive better workplace practices that ripple through the industry. My time in that noisy, gritty paint shop taught me that what starts with a label on a bottle shapes the world in ways that matter far beyond the formula itself.
Understanding the Risks
Butyl acetate brings a fruity aroma and smooth performance to paints, coatings, and many industrial processes. This popularity doesn’t erase the hazards that come with it. Many people overlook basic safety practices around substances that seem familiar. But a solvent like butyl acetate packs a punch. It’s flammable, and the fumes can irritate your eyes, nose, and lungs. Forget to respect these risks, and you invite accidents that can shut down work or even cause injury.
Keeping Fire Out of the Equation
Anybody who’s worked with solvents knows spills and vapor leaks can get serious fast. I remember a facility where a leaky drum of butyl acetate mixed silently with summer heat, the vapors invisible but heavy in the air. All it took was a spark from an old light switch to trigger a small fire that scorched half the loading bay. The real surprise was how quickly it spread. Stories like this remind us that storing butyl acetate near open flames, hot equipment, or even direct sunlight just isn’t worth the risk. The drum or tank must live in a cool, well-ventilated spot, out of the sun and away from anything that could ignite it.
Ventilation Isn’t Just a Suggestion
Breathing butyl acetate for any length of time makes your head spin. Regulations are pretty clear that fumes must be kept below threshold limits set by OSHA and other watchdogs. Storing containers in tight closets or rooms with no airflow means vapor hangs in the air and creates a health hazard. For anyone charged with safety, this means making sure the storage area has reliable ventilation, preferably with fans that push fumes out before they reach people’s noses.
Containers Make a Difference
Using old paint cans or plastic jugs for solvents like butyl acetate may feel thrifty, but the risk isn’t worth a few dollars saved. Compatible, well-sealed metal drums or containers designed for flammable liquids keep vapors from leaking. You also avoid chemical reactions that happen when certain plastics or metals break down in contact with solvents over time. For bigger operations, using proper grounded and bonded storage tanks stops static from sparking fires during transfer or pumping.
Labeling: Not Just for Inspections
Labeling containers doesn’t seem exciting, but it’s a critical step that too many folks skip, especially in a hurry. Clear, durable labels that show what’s inside and list basic hazards protect workers and first responders. In emergencies, there’s no time to guess what’s in that unmarked drum. I’ve watched people panic in a situation only because they didn’t know what they were dealing with.
Spill Kits and Secondary Containment
Accidents happen. Drums leak, valves fail. Facilities that take safety seriously always keep spill kits close by and use secondary containment. This can be as simple as storing drums on spill pallets or inside containment berms. These basics stop one small leak from running into a drain or spreading across the floor.
Training Builds Good Habits
Laws and standards help set the bar, but the best safety culture grows from hands-on training. Workers need to recognize the distinct smell of butyl acetate, know where the nearest eyewash station lives, and understand cleanup procedures. New people make mistakes, but veterans cut corners if they think no one’s watching. Regular refresher courses and peer-to-peer reminders go a long way.
Practical Steps for a Safer Workplace
Storing butyl acetate doesn’t ask for complicated systems or expensive upgrades. Clear rules, good containers, sensible location choices, and regular training make a real difference. In the end, safety isn’t just compliance for its own sake—it’s how you make sure everyone goes home healthy when the job’s done.
What Butyl Acetate Does and Where You Find It
Butyl acetate shows up in a bunch of places—paint thinners, nail polish removers, adhesives, flavors, and fragrances. Walk into a nail salon or a freshly painted room, and you’ve probably breathed some in. Most folks don’t realize that sweet, fruity smell comes from this chemical. It helps dissolve stuff and evaporates fast, so it works well where a quick dry is a selling point.
The Health Side: What Happens If You Breathe It In?
Spending time with high levels of butyl acetate in the air can make your eyes and throat sting or leave your head spinning. Short bursts may not do much more than give you a headache or a brief dizzy spell. Working around it every day, though, can be a different story. People who don’t wear a mask while working long hours with paint or industrial cleaners often walk away with irritated skin, red eyes, or a bad cough. Over time, repeated exposure sometimes messes with your nervous system—think confusion, sluggishness, and trouble keeping your balance.
I once chatted with a carpenter who never put on his respirator, figuring the “strong stuff” wasn’t so bad. After months, he found himself needing breaks just to clear his head—and he’s not alone. NIOSH and OSHA both set pretty strict limits in workplaces because stories like his are a dime a dozen. If you’re working with solvents all day, good ventilation and a pair of gloves make a real difference.
The Environmental Angle
Most butyl acetate that gets into the outdoors comes from industrial spills or factory stacks. It breaks down pretty fast in air—often just a few days—so you don’t see it building up like lead or mercury. That quick disappearance tricks people into thinking there’s no impact. Yet, right after a big spill, plants and fish around the area can still take a hit. High bursts can kill small aquatic creatures and stress out larger ones if the dose is strong enough. Soil and groundwater seem safer, but enough of this chemical dumped in one place can travel and stick around long enough to matter.
Why Facts Matter in Everyday Choices
Lots of folks shrug off these warnings because they figure everyday use can’t be that risky. Yet, the CDC and EPA have flagged enough cases to keep this chemical on safety watch lists. Flammable, heavy vapor, potential to replace oxygen in tight rooms—these aren’t problems that only chemical plant workers face. One leaky can in a garage can make you lightheaded or start a fire. Kids and pets face bigger risks because their bodies react faster and they can’t escape as easily.
Better Ways to Handle Butyl Acetate
We can’t ignore the positives—this solvent keeps products affordable and works well, but that shouldn’t mean cutting corners with safety. Using low-odor, low-VOC products in the house and cracking windows during projects eases most worries. On a bigger scale, industries turning to closed systems, leak detection tech, and recycling more solvent waste throw up fewer red flags for workers and the environment. Even something as simple as reading warning labels and not pouring leftovers down the drain stacks the odds in your favor.
Risk has a lot to do with concentration and exposure time. By sticking with good habits—ventilation, gloves, label checks—homeowners and workers can keep butyl acetate from turning an everyday job into a health emergency. Choosing safer products and calling out bad practices really does lower the chance of someone getting sick or local streams getting polluted. That’s a lesson most folks can relate to as soon as the headache hits.
| Names | |
| Preferred IUPAC name | Butyl ethanoate |
| Other names |
Acetic acid butyl ester
n-Butyl acetate Butyl ethanoate |
| Pronunciation | /ˈbjuːtɪl əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 123-86-4 |
| Beilstein Reference | 1090820 |
| ChEBI | CHEBI:31328 |
| ChEMBL | CHEMBL27717 |
| ChemSpider | 6383 |
| DrugBank | DB02197 |
| ECHA InfoCard | 100.000.606 |
| EC Number | 123-86-4 |
| Gmelin Reference | 1907 |
| KEGG | C00596 |
| MeSH | D001973 |
| PubChem CID | 31272 |
| RTECS number | AF7350000 |
| UNII | NLQ431P6UV |
| UN number | UN1123 |
| Properties | |
| Chemical formula | C6H12O2 |
| Molar mass | 116.16 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | fruity |
| Density | 0.882 g/cm3 |
| Solubility in water | 0.68 g/100 mL (20 °C) |
| log P | 1.82 |
| Vapor pressure | 11.5 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 25 |
| Basicity (pKb) | 13.27 |
| Magnetic susceptibility (χ) | -8.76E-6 |
| Refractive index (nD) | 1.394 |
| Viscosity | Viscosity: 0.74 mPa·s (at 25 °C) |
| Dipole moment | 1.84 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 266.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −479.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2678.4 kJ/mol |
| Pharmacology | |
| ATC code | D01AE19 |
| Hazards | |
| GHS labelling | **GHS02, GHS07** |
| Pictograms | `GHS02, GHS07` |
| Signal word | Warning |
| Hazard statements | H226, H336 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-3-2 |
| Flash point | 27°C (81°F) |
| Autoignition temperature | 670 °F (354 °C) |
| Explosive limits | 1.7% - 7.6% |
| Lethal dose or concentration | LD50 oral rat 10,768 mg/kg |
| LD50 (median dose) | LD50 (median dose): 10,768 mg/kg (rat, oral) |
| NIOSH | **NA-1165** |
| PEL (Permissible) | 150 ppm |
| REL (Recommended) | 150 ppm |
| IDLH (Immediate danger) | 1700 ppm |
| Related compounds | |
| Related compounds |
Ethyl acetate
Isobutyl acetate Propyl acetate Methyl acetate Butanol Acetic acid |



