Ammonium Acetate: From Laboratory Staple to Industry Workhorse
Historical Development
Ammonium acetate traces its use back to the days of classic chemistry when early scientists needed simple salts to explore acid-base behavior. Chemists reached for ammonium acetate in the late 18th century to prepare buffer solutions before the concept even had a name. The salt’s stability and versatility charmed lab workers, finding a home in analytical procedures, textile dyeing, and even early preservation methods. Through the 20th century, its production ramped up. High-purity grades joined the line-up as analytical chemistry, environmental science, and pharmaceuticals demanded consistent quality. Its journey through time shows how a single compound can quietly underpin decades of progress without ever stealing the spotlight.
Product Overview
Ammonium acetate usually shows up as a white, hygroscopic solid. You find it in bottles tucked away on shelves at universities, but it also appears as granular or crystalline material for use in bulk industry. No frills, but highly effective where it counts: as a buffer in labs, as a reagent for organic synthesis, and as an agent to speed up certain industrial processes. Though often not the main ingredient in a given blend, omit ammonium acetate and many procedures just grind to a halt. The material’s broad compatibility puts it in demand across pharmaceuticals, agriculture, water treatment, and biochemistry.
Physical & Chemical Properties
Ammonium acetate’s formula is NH4CH3COO. At room temperature, it stands as a white solid, readily dissolving in water and alcohol. Its melting point hovers around 114°C, though the salt decomposes at higher temperatures, releasing ammonia and acetic acid vapors. This property makes storage conditions important — keeping it in tightly sealed containers away from high heat or open air preserves quality. It reacts as a weak electrolyte in water, making it ideal for creating buffer solutions with a neutral pH. Ammonium acetate’s volatility at elevated temperatures sometimes benefits chemical processes where removal of the buffer residue is necessary, such as in certain mass spectrometry workflows.
Technical Specifications & Labeling
On the market, labels specify purity grades. Lab reagent and analytical grades demand near-perfect compositions, while technical grades provide cost-effective options for bulk users. Specifications usually cover appearance, moisture content, pH range for aqueous solutions, chloride and sulfate content as impurities, and the absence of heavy metals. Manufacturers follow regulatory standards such as USP, ACS, or ISO norms—highlighted on packaging so users know exactly what they’re getting. Documentation covers lot traceability, recommended storage (cool, dry, sealed), and expiration for best results. These practices provide traceability and support responsible usage in applications sensitive to impurities or contamination.
Preparation Method
Ammonium acetate springs from the neutralization of acetic acid with ammonia. Producers either bubble gaseous ammonia through glacial acetic acid or mix aqueous forms of both. Careful control over stoichiometry and temperatures prevents side reactions, like the formation of ammonium carbonate. After mixing, the solution is evaporated to crystallize the salt, and subsequent drying delivers the final product. Some operations add steps to remove residual water or acetic acid, which tightens purity specs for laboratory and pharmaceutical use. From large-scale reactors to bench-top batches, the core chemistry remains direct—a testament to the straightforwardness of classical salt synthesis.
Chemical Reactions & Modifications
Ammonium acetate doesn’t limit itself to acting as just a buffer in reactions. Add it to certain organic syntheses—such as the preparation of acetamides or as a source of ammonia in the Leuckart reaction—and it participates directly. In the laboratory, heating ammonium acetate yields acetamide and water via dehydration. It decomposes at elevated temperatures, providing routes to gaseous ammonia and acetic acid. The salt reacts with strong acids and alkalis, making it suitable for ion-exchange studies and related applications. Occasionally, researchers modify ammonium acetate by blending it with chelating agents for specialized chromatography, enhancing its selectivity toward certain ions or molecules.
Synonyms & Product Names
While most know it as ammonium acetate, catalogs and research papers sometimes refer to it as ammonium ethanoate or simply “ammonium salt of acetic acid.” In German, you’ll see “Essigsaures Ammoniak.” Certain brands attach trade names to highlight purity or specialty preparation, but underlying substance remains the same. Having lived with chemical catalogs and research article searches, it’s clear these synonyms trip up newcomers—making cross-checking CAS numbers (631-61-8) essential for accurate sourcing. Calling it by the wrong name doesn’t change its chemistry, but mixing up formulations can lead to failed experiments or process breakdowns.
Safety & Operational Standards
Safety sheets list ammonium acetate as a compound with low acute toxicity, but prolonged exposure to dust or high concentrations can irritate skin, eyes, and mucous membranes. Spills need simple containment and cleanup, as water can dissolve most residues. Inhalation of dust is best avoided with basic controls—ventilation, masks, and gloves in bulk handling settings. Fire risk remains low, though like many ammonium salts, heating to decomposition releases ammonia fumes, which require inhalation precautions and good ventilation systems. Companies take these standards seriously, training staff on good housekeeping and handling practices to reduce accidental exposures or cross-contamination. Proper labeling and secure storage help keep things safe for workers and researchers.
Application Area
The impact of ammonium acetate stretches across industries. Chromatographers reach for it to regulate pH in mobile phases. Environmental labs use it in soil extraction protocols. Biochemists pick it for protein and DNA precipitation, mass spectrometry, and buffer preparation. In agriculture, it sometimes serves as a nitrogen source in specialty fertilizers. Textile processors employ it as a mordant and for dye fixation, especially on protein fibers. Pharmaceutical manufacturers appreciate its volatility, allowing easy removal after synthesis steps—avoiding residue that could affect drug safety or efficacy. Experienced users value ammonium acetate’s flexibility and its performance where results depend on tight chemical control.
Research & Development
Ammonium acetate’s popularity as a buffer and intermediate keeps it front-and-center in labs pushing the boundaries of analytical chemistry. In LC-MS workflows, researchers pick ammonium acetate for its low background signal and volatility, both critical when chasing trace analytes or working with sensitive detectors. Biotechnologists test its effect on protein folding and stabilization, tracking subtle conformational changes. Environmental scientists rely on it to pull out challenging cations from soils or water samples, improving precision in contamination studies. R&D teams explore tweaks to purity, particle size, and packaging to meet changing needs, especially as analytical methods move toward miniaturization and automation.
Toxicity Research
Toxicologists agree that ammonium acetate doesn’t pose the acute toxic threats of many industrial chemicals. Studies in rodents show relatively high LD50 values, which speaks to its low inherent toxicity. Long-term exposure at high levels, especially in poorly ventilated spaces, can cause respiratory and mucosal irritation or nausea. Researchers look at ammonium and acetate ions separately because chronic overexposure to either, particularly in those with kidney or liver impairment, could contribute to metabolic imbalances. Environmental impact debates sometimes pit ammonium acetate against longer-lived salts—fortunately, its ready breakdown in soil and water through natural processes limits risks, provided good waste management practices remain in place.
Future Prospects
Ammonium acetate continues to hold a spot as chemistry and materials science expand into new territory. The rise of green chemistry drives demand for reagents that decompose cleanly without toxic byproducts. Its role in advanced chromatography grows as analysts chase even finer levels of detection. With more biopharmaceuticals entering the market, production facilities seek out highly purified salts to conform with regulatory demands. As environmental regulations tighten, even waste salt recovery and recycling get a closer look, pushing producers to refine manufacturing and reclamation methods. The steady march of automation and digitized lab workflows means packaging and documentation will keep evolving—but the chemistry behind ammonium acetate remains as relevant as ever.
Key Ingredient in Research Labs
Think of ammonium acetate as a familiar face across chemistry classrooms and professional labs. Chemists use it for making buffers. These mixtures help keep a steady pH, especially during sensitive reactions and separations. When I started out in the lab, nothing helped more than a simple ammonium acetate buffer for high-performance liquid chromatography (HPLC). It helps keep samples stable so test results stay reliable, and it mixes well with both water and common organic solvents like methanol.
Synthesis in Drug Manufacturing
Drug companies look at ammonium acetate as a basic building block. Pharmaceuticals often demand clean, precise reactions, and this compound helps make that possible. It acts as a safe source of both ammonia and acetate during synthesis. Many medications or active ingredients in over-the-counter drugs start out with steps that include ammonium acetate — from simple antipyretics to antibiotics. According to the National Institutes of Health, certain drugs depend on acetate ions for proper formulation, and ammonium acetate delivers without introducing unwanted metals or contaminants.
Food Testing and Quality Control
Imagine food safety experts checking the freshness of milk or cheese. Ammonium acetate works as a reagent for confirming ingredients in foods or testing for adulteration. High purity matters in this setting. When I worked alongside a food technician, she often used ammonium acetate during titrations or as a buffer in spectrometric analysis. This helps spot impurities or deceptive additives before products hit store shelves.
Proteomics and Genomics
Researchers investigating proteins or genetic material often turn to this compound. Mass spectrometry, a core method in biology, often relies on ammonium acetate to keep proteins and peptides from clumping together. It helps ions move smoothly so machines can detect and analyze them with clarity. In genomics, it helps remove proteins from DNA samples so genetic testing can run without interference. Scientists value its role because it leaves behind little residue and can be removed easily afterwards.
Soil and Environmental Analysis
In agriculture and ecology, ammonium acetate plays a big part in testing soil. Farmers depend on these tests to decide on fertilizer use and to monitor soil health. Soil scientists use ammonium acetate to help extract minerals like potassium and calcium. The process gives a clear snapshot of available nutrients. Good soil supports healthy crops, and this routine testing keeps yields strong year after year. The USDA often refers to ammonium acetate extraction as a gold standard for reporting soil fertility.
A Solution for Textile and Dye Industries
Textile manufacturers deal with dyes and fabric treatments where pH makes or breaks color outcomes. Ammonium acetate acts as a pH controller, making sure colors bond properly to fibers. I’ve seen it used to help brighten fabric dyes and reduce waste in large-scale operations. Better pH control means richer colors and less chemical runoff, which supports cleaner production.
Handling and Looking Ahead
While ammonium acetate offers versatility, handling it with care matters just as much. Even routine compounds like this require solid training and proper ventilation. It works safely in most lab conditions but produces ammonia if heated in closed spaces. As science and technology push for greener methods and precise analytics, the low toxicity and reliable behavior of ammonium acetate make it a standard in practical workflows, from the research bench to the fields and factories.
Why It Matters to Handle Ammonium Acetate Right
Plenty of labs and classrooms stock ammonium acetate. It’s in bottles on chemical shelves or tucked away in cold rooms, helping folks with chemistry, pharmaceuticals, or testing soil. Anyone who’s spent time with this stuff knows it wears labels loaded with warnings and storage advice. People trust it to get the job done, but ignore the basics and that bottle can ruin an experiment or even pose risks nobody saw coming.
Shelf Life: Don’t Rely on Old Stock
I’ve seen enough bottles of ammonium acetate build up dust over the years to know one thing: it doesn’t last forever. Stored wrong, it clumps, cakes, and can start smelling off. Manufacturers usually set the shelf life between two to three years under ideal conditions, which means a dry, tightly sealed container out of the sun’s way. Even sealed, humidity can work its way in, so old bottles past their expiration date don’t always deliver the results people expect.
What most folks miss is that ammonium acetate absorbs water right out of the air. Let it get humid, even for a day, and the powder can turn damp or hard. I’ve opened containers forgotten at the back of the shelf that had fused into one sticky mass. If you’re working in a research setting—or worse, mixing up buffer solutions for something sensitive—using degraded chemicals means wasted time, money, and trust.
Simple Storage Rules That Make a Difference
Consistent success in the lab, or anywhere ammonium acetate is used, starts with proper storage. A cool, dry room—think 15°C to 25°C—matters more than most realize. I’ve seen folks stash bottles in fridges and freezers convinced it’ll extend shelf life. Cold can help, but if the container isn’t moisture-proof, condensation will sneak in and cause bigger problems.
Always seal the container tightly after grabbing what’s needed. Even a few minutes open in a humid room spells trouble. Dedicated desiccators give another layer of protection, keeping out moisture between uses. If the budget allows, stock chemicals in small bottles to avoid exposing the whole stock to air over and over.
What Happens When Storage Goes Wrong
Besides forming clumps or dissolving in its own absorbed water, ammonium acetate degrades and can break down completely, losing potency. That means failed reactions and wasted samples. Worse, chemical breakdown can produce acetic acid or ammonia vapors, adding safety hazards. The odor may seem minor, but in a closed room, it burns the eyes and nose and sets off alarms. Flammable and corrosive hazards crop up fast with poor practices.
What’s Worked for Me—and for Colleagues
I’ve always kept logs on chemical inventories, marking received and opened dates with a thick marker right on the label. Some departments set reminders in their phones to review stocks every quarter, tossing anything suspicious or out of date. More than once I’ve saved a tight deadline by sticking this habit—no panicky rush to reorder after a failed run.
Bulk orders seem cheap, but it rarely pays off unless chemicals move quickly. Going for smaller containers—even if the price-per-gram goes up—keeps things fresh. Communicate with colleagues so everyone knows the routine and does their part to check for caked, cracked, or off-smelling stock. Basic vigilance in these simple steps means fewer failed tests and a safer, smoother day for everyone handling ammonium acetate.
Digging Into Everyday Use
Ammonium acetate shows up in places most folks don’t think about—labs, food processing plants, and sometimes classrooms. People use it to set up chemical buffers, preserve meat products, and even de-ice runways. A lab tech once told me he had handled ammonium acetate a hundred times through his career. Yet the label on the jar always spells out: “Caution—handle with care.” This gives you an idea why people keep asking about safety.
Looking At Toxicity
Holding a degree in chemistry taught me that toxicity comes down to dose and exposure. Ammonium acetate isn’t close to the most dangerous chemical in a lab but ignoring its effects never makes sense. According to the U.S. National Library of Medicine, ammonium acetate can cause irritation to eyes, skin, and the respiratory tract. Swallowing a large amount will cause nausea, vomiting, and even confusion. If you’re handling it for work, it’s not smart to skip gloves or eye protection.
Breathing in a little won’t cause immediate harm. Still, accidents where dust fills the air feel different. Working in small rooms, I’ve felt the air get thick with chemicals. Shortness of breath and coughing send a clear signal—open windows, get a fan, put on a mask, and try to keep containers closed when possible. The best habit I’ve picked up is washing hands and changing out of lab coats before eating.
Environmental and Workplace Concerns
What happens after ammonium acetate leaves the package holds just as much weight. Wastewater loaded with ammonium salts can have a field day on nearby waterways, increasing algae growth and upsetting oxygen levels for fish. OSHA and the EPA both provide straightforward guidance for preventing spills and disposing of solutions—never pour leftovers down the drain, keep storage dry, and label everything. These steps sound simple, but skipping them leads to polluted water or even injured animals.
The Food and Drug Administration allows small amounts of ammonium acetate in foods, so the chemical finds its way onto processed meats and pickled products. Those who study food safety know: in regulated amounts, it doesn’t raise alarms. Eating large amounts straight from the container, though, puts someone right at the edge of acute illness. Most people rarely go near pure ammonium acetate, so the real risk sits with those in industry or labs.
Finding Smarter Practices
I always encourage people to read up and gear up before starting any project. Lab managers say the same thing: “Know your chemicals, check the data sheets, and don’t cut corners.” Having proper ventilation and personal protective equipment on hand makes a difference. Spills should lead to immediate cleanup, not tomorrow’s problem. Those handling ammonium acetate outside of chemistry labs often get safety lessons before they step foot on the floor, and it pays off to listen.
Companies and schools have a responsibility to update safety protocols and make sure everyone actually follows them. Every year, reports show a handful of avoidable chemical accidents in settings where the rules looked good on paper but nobody paid attention. The need for good habits won’t ever go away, no matter how common or “safe” a chemical appears.
Keeping Perspective
Ammonium acetate shouldn’t scare people, but it deserves respect. If someone lives or works with chemicals, trusting labeled safety precautions, monitoring exposure, and thinking about downstream effects becomes second nature. Overlooking even a simple salt like this can bring trouble, but solid routines keep those risks away from daily life.
A Look at Its Core
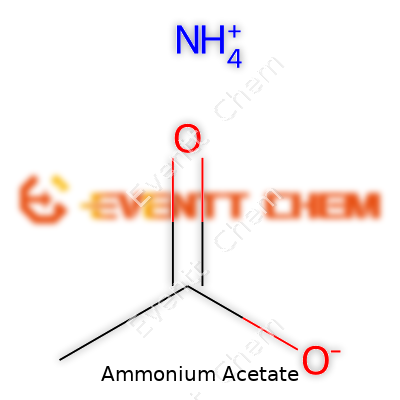
Ammonium acetate isn’t some elusive chemical only found in science labs. It pops up in everyday life, hiding in products like food additives and pharmaceuticals. The formula seems basic: C2H7NO2. Its molecular weight hits 77.08 grams per mole. At a glance, those numbers look dry, but they speak volumes for anyone working with this compound.
Molecular Details and Why They Matter
Breaking down the formula gives a glimpse into its structure. Two carbons, seven hydrogens, one nitrogen, and two oxygens come together for a reason. The ammonium part (NH4+) bonds with acetate (CH3COO-). This combo forms a salt that dissolves easily in water. That trait makes ammonium acetate a useful buffer in labs and manufacturing.
Molecular weight isn’t just a number on a sheet. It decides dosing and mixing, especially when precision keeps products safe, whether we're talking IV solutions or processed food. No one wants a miscalculation in a healthcare setting. That extra decimal after 77 can affect reactions and patient outcomes.
From Science to Everyday Uses
I still remember the first time I used ammonium acetate in a college chemistry course. Even in basic buffer experiments, one mistake in weighing led to results that didn’t add up. Turns out, understanding the real-world implications of chemical properties sometimes comes from cleaning up a fizzing mess on the lab table.
Practical chemistry values these building blocks. Food technologists use ammonium acetate to balance acidity in products. Analytical chemists rely on its buffering powers for high-performance liquid chromatography. Pharmacists work with its properties to stabilize medicines. Getting the formula wrong doesn’t just mess up paperwork; it can set back research or affect shelf life.
Facts, Risks, and Solutions
With every chemical comes responsibility. Handling ammonium acetate demands respect. It’s not dangerous in small doses, but exposure to the eyes or inhalation can irritate. Manufactures list it by the correct molecular formula for a reason—regulations ask for it, and safety depends on it.
Some manufacturing plants have struggled to stick with up-to-date labeling practices. Lab workers and technicians must check the Safety Data Sheet (SDS) before handling. Industry groups recommend double-checking molecular weights on every batch. Training staff in chemical math seems simple, but it’s saved more than a few headaches. It’s not just about ticking off legal boxes. Knowing how much to add, how to store it, and when to throw out expired stock builds trust between suppliers and users. Transparency matters, especially for products bound for the public.
Building Trust Through Shared Knowledge
The molecular formula and weight may look like fine print, but they spell out safety and reliability in each gram. A small mistake can ripple into everything from hard-to-digest snacks to shelf-stable medicines. Clear communication from producer to end user shapes trust. Quality demands it; so does anyone taking these products home or to work.
So next time you see C2H7NO2 on a label, know real science and careful calculation stand behind those letters and numbers.
Why Safe Handling Matters
Working in labs and industrial settings, I’ve seen that many chemicals earn a reputation for being trickier than they look. Ammonium acetate lands in this category. It’s commonly used in chemistry labs, food processing, and even pharmaceuticals. At first glance, it doesn’t scream danger, but it doesn’t mean it belongs in a casual storage shelf next to the lunchroom coffee.
Ammonium acetate can release fumes that cause eye, nose, and throat irritation. Anyone who’s spent a morning sorting through unlabeled bottles knows the sting from a forgotten container. Spills become slippery, turning a routine task into an accident scene if people don’t wear proper shoes or rush without gloves.
How to Store and Handle It
Keep ammonium acetate in tightly sealed containers. Moisture in the air loves to sneak in and turn the salt into a lumpy mess, so a dry spot with limited humidity keeps things easy to manage. I’ve learned the hard way that storing chemicals above shoulder height invites trouble—spills and eye exposure grow more likely. Ground-level shelves with clear labels go a long way toward safer workspaces.
Personal protective equipment isn’t just for dramatic photos in safety manuals. Goggles, lab coats, and gloves should go on before a single container opens. I remember a colleague who brushed off gloves as unnecessary for “simple salts” until he developed a rash after an unexpected leak. Skin contact might seem harmless, but the repeat exposure builds up over time.
Ventilation: Keeping Air Clear
Good air flow keeps ammonium acetate use safer. Fume hoods aren’t just boxes that get ignored—they pull vapors away so no one ends up with red eyes or a sore throat after routine work. Anyone handling the powder form needs to avoid inhaling dust clouds. Even opening jars should happen gently, never in haste.
Why Reckless Disposal Causes Trouble
Tossing ammonium acetate down the sink never sat right with me. I’ve seen waste bins, drains, and even storm sewers misused out of convenience. Ammonium ions add up in water sources, feeding algal blooms and other environmental headaches. Streams can’t work as free-for-all chemistry labs. Waste contractors exist for a reason.
Containers and waste with trace chemicals should go into dedicated hazardous waste bins. I always separate inorganic salts from the general trash, avoiding cross-contamination and surprise reactions during later disposal rounds. Rinsing glassware with plenty of water after work makes later cleaning less of a chore and stops acids from forming unexpectedly.
How Safety Becomes a Habit
Good training goes beyond reading rules posted on a wall. Staff briefings, reminders, and regular walk-throughs spot habits slipping out of line before mistakes happen. Everyone, from new hires to seasoned techs, needs refresher talks about spill kits and first-aid procedures.
Keeping an updated safety data sheet nearby saves time during confusion. Emergencies don’t wait for someone to Google instructions. Posting these sheets in visible spots—right beside storage shelves or sinks—means answers come quickly when needed most.
Smarter Solutions
Labs and companies that invest in better storage containers, clear labeling, and routine staff training cut down on errors and accidents. Looking for greener alternatives or using the lowest effective amount of ammonium acetate lightens the waste load. Teamwork, communication, and smart infrastructure keep everyone safer and the environment cleaner.

| Names | |
| Preferred IUPAC name | azanium acetate |
| Other names |
Acetic acid, ammonium salt
Ammonium ethanoate |
| Pronunciation | /əˌməʊniəm əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 631-61-8 |
| 3D model (JSmol) | `3D molecular model (JSmol) string for Ammonium Acetate:` `NH4C2H3O2` |
| Beilstein Reference | 1718733 |
| ChEBI | CHEBI:62947 |
| ChEMBL | CHEMBL1377 |
| ChemSpider | 3796 |
| DrugBank | DB04382 |
| ECHA InfoCard | 03b7bb0e-6b30-4492-97bc-7ae5fa83e473 |
| EC Number | 204-822-2 |
| Gmelin Reference | 816 |
| KEGG | C01717 |
| MeSH | D000648 |
| PubChem CID | 7004 |
| RTECS number | AF3675000 |
| UNII | 7U0877458F |
| UN number | UN1841 |
| CompTox Dashboard (EPA) | DTXSID2020639 |
| Properties | |
| Chemical formula | NH4C2H3O2 |
| Molar mass | 77.08 g/mol |
| Appearance | White crystalline powder |
| Odor | Slightly acetic |
| Density | 1.07 g/cm³ |
| Solubility in water | Very soluble |
| log P | -2.32 |
| Acidity (pKa) | pKa ≈ 4.76 (acetic acid), pKa ≈ 9.25 (ammonium ion) |
| Basicity (pKb) | 8.80 |
| Magnetic susceptibility (χ) | -44.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.409 |
| Viscosity | 1.8 mPa·s (at 25 °C) |
| Dipole moment | 5.9 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 221.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −739.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | −1010.3 kJ/mol |
| Pharmacology | |
| ATC code | B05XA15 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes eye and skin irritation. |
| GHS labelling | GHS07, GHS Hazard Statement: H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | > 607 °C (1125 °F; 880 K) |
| Lethal dose or concentration | LD50 oral rat 7360 mg/kg |
| LD50 (median dose) | LD50 (median dose): 736 mg/kg (oral, rat) |
| NIOSH | B0080 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Ammonium chloride
Sodium acetate Ammonium carbonate Ammonia Acetic acid |



