Aluminum Acetate: An In-Depth Commentary
Historical Development
Aluminum acetate has roots that reach back to early attempts at textile dyeing and wound care, standing out as a compound that chemists, pharmacists, and fabric workers learned to trust for both its astringent properties and buffering behavior. Early records show apothecaries mixing simple vinegar and aluminum salts for dressings, long before regulatory labels existed. Its place in medicine and industry built up over time, a result of hands-on necessity. As chemical understanding deepened, aluminum acetate found a place in standard pharmacopoeias, often known by trade names like Burow’s Solution—named after German military surgeon Karl August Burow in the 19th century, who noticed its calming effect on inflamed skin. The adoption of synthetic methods that provided greater purity and consistency in the 20th century expanded its reach, paving the way for broader medical and laboratory applications.
Product Overview
Marketed under labels such as Burow’s Solution and Domeboro, aluminum acetate shows up most often as a topical astringent. When I’ve picked up a bottle at the pharmacy, most labels point to its use for minor skin irritations, insect bites, and even as a soak for athlete’s foot. While the main ingredient is the basic aluminum acetate complex, many over-the-counter products include small amounts of stabilizers. Its character as both an astringent and mild antiseptic lets it cross boundaries from pharmacy shelves to textile labs and water treatment facilities.
Physical & Chemical Properties
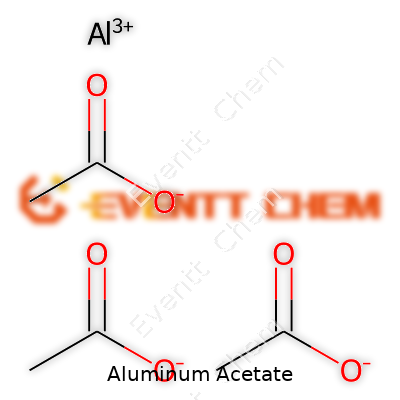
Pure aluminum acetate shows as a white powder or crystalline solid, hygroscopic in nature—absorbing water from humid air and easily dissolving in it. It sometimes appears as a syrupy liquid in concentrated solutions. The compound doesn’t give off a strong smell, which makes it easier to use in wound care. Solubility in water stands out, with solutions forming mildly acidic pH, a quality important to its soothing effect on skin and its use as a buffer. Chemically, its key feature lies in the basic aluminum ion bound to acetates and hydroxo groups, allowing weak cross-linking reactions that tighten cell membranes—hence the astringent action. The structure, Al(C2H3O2)3, reacts with both acids and bases, a trait that sets the stage for its varied modifications.
Technical Specifications & Labeling
Regulations require clear labeling of aluminum content, acetate concentration, and preparation method for medicinal or food-related applications. In my own experience reading through pharmacopeia and regulatory documents, U.S. regulations demand specifications for aluminum acetate solution with aluminum between 4-5%, acetic acid content by weight, and a requirement for sterility in wound and eye products. Labels also call out potential allergens and additives. Packages warn users not to ingest these external preparations, and some countries limit solution strengths to protect against irritation. Product sheets for industrial uses list pH ranges and suggest guidelines for storage temperature, reflecting the compound’s tendency to degrade in high heat or humid conditions.
Preparation Method
Home chemistry sets once used simple recipes: acetic acid combined with aluminum hydroxide or aluminum sulfate, mixed under gentle heat. Commercial processes lean on controlled chemical reactions. In labs, aluminum sulfate reacts with lead acetate, filtering out any insoluble salts, yielding a clear solution or drying down to a crystalline product. The resulting substance requires careful washing to remove excess acetic acid and any heavy metal traces, ensuring safety for both wound care and chemical applications. Modern processes use purified starting materials to cut contamination risk, and equipment made of non-reactive glass or plastic holds up best during mixing and storage.
Chemical Reactions & Modifications
Aluminum acetate behaves as a weak acid, sensitive to other ions in a mixture. In cleaning up wastewater or softening textiles, it forms insoluble compounds with phosphates and sulfates. In the lab, it takes part in exchange reactions with other acetates and buffers. Add more acetic acid, and you get a more acidic solution; shift the balance toward alkali, and it hydrolyzes to aluminum hydroxide, losing its astringent properties. The acetate group can be replaced with other carboxylates to change solubility or reactivity. These modification routes open doors for specialty uses—flame-retardant textiles, photographic chemistry, and even novel drug delivery materials.
Synonyms & Product Names
This compound wears multiple names, depending on where it’s sold and for what purpose. Pharmacists recognize “Burow’s Solution,” “Aluminum diacetate,” or simply “Alum Solution.” The textile industry sometimes tags it as “aluminum ethanoate.” Domeboro and generic “aluminum acetate soak” also appear on drug store shelves. Catalogs for academic and industrial chemicals keep things formal, using “aluminum(III) acetate.” This patchwork of names means a buyer has to read closely—mistaking basic for neutral aluminum acetate, or vice versa, can throw off experimental results or medicinal outcomes.
Safety & Operational Standards
Handling aluminum acetate doesn't usually require heavy protection, but powder can irritate eyes and lungs, and concentrated solutions produce mild skin irritation. Product datasheets warn of overuse on broken skin or mucous membranes, especially in people sensitive to aluminum. In a clinical setting, protocols direct care workers to use single-use applicators to avoid contamination. Disposal practices keep waste solutions out of municipal water systems when possible. Ignoring storage guidelines—like leaving solutions unsealed—leads to hydrolysis or loss of effectiveness. Regulatory agencies in Europe and North America expect proof of manufacturing controls and routine product quality assurance, meeting not just safety but also environmental standards.
Application Area
I’ve seen aluminum acetate’s reach go well past basic wound washes: podiatrists use it to reduce swelling in foot infections, dermatologists recommend it for eczema flares, and veterinarians employ it to clean animal wounds. Its mild astringency soothes rashes and relieves itching, making it a staple during summer hikes surrounded by poison ivy. Beyond skin care, researchers utilize it as a mordant in textile dyeing, locking dyes into cotton fibers. Industrial water treatment stations add it to help neutralize acid waste or clear up cloudy water through flocculation. Some chemists use it as a precursor in preparing more complex aluminum-containing catalysts and materials, recognizing its adaptable chemical backbone.
Research & Development
Recent years brought a push to optimize aluminum acetate for quicker, less irritating healing. Scientists work to combine it with plant extracts or antimicrobial agents, targeting stubborn skin infections or chronic wounds. Textile scientists explore its role in eco-friendly dyeing, searching for less polluting alternatives. Some studies look into nano-formulations—shrinking particle size to improve penetration through the skin, with mixed results so far regarding both safety and efficacy. Green chemistry aims to swap out traditional synthetic routes for biomass-derived acetic acid and lower-energy processes, striving to meet stricter environmental guidelines. In a crowded marketplace, companies invest in more accurate sensors for concentration and pH to guarantee consistent product performance.
Toxicity Research
Aluminum compounds draw scrutiny for possible neurotoxic effects, especially with long-term exposure. Most research indicates that topical aluminum acetate, when used as directed, results in minimal absorption and low risk. Swallowing large quantities, as in accidental ingestion by children or animals, brings risk of gastrointestinal irritation and, in rare cases, aluminum toxicity. Animal studies hint that solutions above 5% can damage sensitive skin, so manufacturers keep concentrations below this level. Ongoing post-market surveillance and periodic clinical reviews track any emerging side effects. Regulatory agencies periodically review new toxicity and exposure data, adjusting approved labeling and warnings as needed.
Future Prospects
Aluminum acetate’s adaptability promises a solid future. Researchers continue to look for ways to improve delivery in both medical and industrial applications, pursuing longer-lasting formulations and better compatibility with modern wound dressings. Opportunities exist for greener production, better recycling of aluminum-containing waste, and smart new uses in environmental cleanup. Advances in analytical chemistry could unlock detailed understanding of reaction mechanisms, leading to new modifications or safer alternatives where allergies or sensitivities persist. The compound’s long history and dependable track record in so many fields suggest it will remain relevant, especially as scientists uncover fresh problems for it to help solve.
A Familiar Face in Healthcare
Walk into any pharmacy and you’re likely to find aluminum acetate tucked in the first-aid aisle. Most people bump into it as a clear liquid labeled “Burow’s Solution” or “Domeboro Soak.” Doctors and old-school nurses swear by it for dealing with itchy, irritated skin. It’s been around for generations, and for good reason.
Real Relief for Skin Troubles
Rashes, poison ivy, sweating feet, bug bites, and even that stubborn heat rash that flares up in the dead of summer—aluminum acetate has been brought out to help calm them all. I grew up spending summers in the woods, and there was always a bottle in the medicine cabinet. After a long hike through poison ivy, soaking a towel and pressing it onto my arms would stop the almost unbearable itching.
Aluminum acetate works because it draws moisture out of inflamed areas. Dermatologists call this an “astringent” effect. It dries out weepy or oozing rashes and keeps bacteria from having an easy time spreading. The drying doesn’t sting in the way alcohol does. It’s actually soothing, and the relief comes pretty quick—ask any coach or parent who’s dealt with sweaty feet or athlete’s foot in teenagers. The same logic applies to other skin conditions: by cutting down on swelling and keeping things clean, skin can heal.
What Science Says
Medical guidelines point to aluminum acetate as a reliable, over-the-counter treatment for minor wounds, insect bites, and eczema. Mayo Clinic and Cleveland Clinic articles both list it as a safe option for itchy, moist skin problems. It has a low risk of causing side effects, though doctors do warn to keep it out of eyes and not to swallow it. Rashes from poison ivy or poison oak clear up faster with regular soaking.
It’s not just about convenience or tradition—clinical studies highlight how astringents like aluminum acetate reduce inflammation and itching. It’s even on the World Health Organization’s list of essential medicines for local skin care.
Outside the Medicine Cabinet
While health is the main story, industries haven’t ignored aluminum acetate. Textile workers, especially in specialty fabric production, have used it to fix dyes to cloth. Gardeners occasionally use it to treat plant diseases, taking a page from the same wound-care playbook.
Cosmetics companies have also put it into deodorants and antiperspirants. Its sweat-blocking power meets demand for dry and comfortable skin. Personal experience lines up with the research—people with really sensitive skin sometimes react less to these formulas.
Room for Caution and Smart Use
Like anything that dries and cools, aluminum acetate can tip from helpful to irritating if overused. Dry skin gets itchy and cracked, so moderation matters. It shouldn’t go on deep cuts or be left soaking for hours on end. Some skin conditions need real medical care and not just a home remedy. As always, if something looks infected, getting advice from a doctor is the safer route.
Taking care of small skin complaints helps more than comfort—it prevents bigger problems. Products like aluminum acetate hold their value in a world packed with complicated treatments. They’re simple, proven, and easy to get. Instead of promising miracles, they offer practical relief where it’s needed most.
Aluminum Acetate Solution in Real Life
Most folks don’t walk around thinking about aluminum acetate until a rash pops up or an itch refuses to quit. Once, after a summer hike landed me with a nasty patch of poison ivy, my pharmacist pointed to this clear, almost odorless liquid and called it a game changer. A lot of people know it by its common brand name, Domeboro®, and it usually sits in the first aid aisle, not far from bandages and calamine lotion. The science behind it isn’t complicated: aluminum acetate soothes angry skin. Big names in dermatology and pharmacy back its use. Data from Mayo Clinic and American Academy of Dermatology show its safety record stays strong over decades of use.
Where and Why People Use It
The uses aren’t just limited to poison ivy. Athletes with sweaty feet, kids who get bug bites all summer, folks dealing with eczema or even stubborn underarm redness—aluminum acetate steps in for all of these. What’s striking is how it draws out moisture without burning or stinging. The mild astringent effect dries weepy rashes, calms inflamed spots, and helps skin recover faster. Plenty of busy parents keep it on hand after learning how fast it kicks in for heat rash or allergic reactions from nickel jewelry.
Here’s How I’ve Used It (And How Pros Suggest You Do It)
The process stays simple: soak one or two packets or tablets in cool water, then either soak a cloth and lay it over the rash or dab the mix with clean cotton balls. During my worst poison ivy run, cool compresses every four hours made a world of difference. No sticky mess, no harsh smell—just a clean, soothing feel. Boards like the American Pharmacists Association recommend up to four times a day, making sure the skin dries between applications. Don’t scrub or rub; gentle dabbing does the trick. Some people use it as a foot soak for athlete’s foot, which knocks down the irritation and gives relief in a few days.
Risks and Limitations
Rarely, a few might see their skin dry out or flake after several uses. Anyone allergic to aluminum compounds should steer clear, and open wounds shouldn’t see this stuff at all. Pediatricians agree that infants under 6 months should avoid it unless a physician says otherwise. If redness keeps spreading, or there’s pus, that could mean an infection—time to get medical advice. These instructions show up on reputable health sites and reflect both historical data and updated medical guidance.
Why It Matters and Possible Next Steps
This solution matters most when over-the-counter creams don’t do enough, or if something non-greasy fits better into a routine. Emergency rooms sometimes suggest it for minor burns before more complicated treatments. Community pharmacists often teach parents and coaches how to use it with confidence. Better labeling could help people avoid simple mistakes like making mixtures too strong, which dries out the skin too much.
Many pharmacies aim to educate, but online resources can go further. Volunteer local health educators could spread detailed, plain-language guides for families with children prone to skin rashes. Clinics might offer handouts with drawings to explain each step for those facing language barriers, so everyone stays safe and gets the most out of an old but reliable bottle.
Aluminum acetate solution may never make headlines, but in any bathroom cabinet or gym locker, it quietly earns trust by providing relief without hassle. Families and healthcare teams that know how to use it well see fewer miserable, itchy weeks—and that stands out as a small but real win.
Making Sense of Aluminum Acetate
Aluminum acetate steps onto drugstore shelves mostly as a remedy for rashes, itching, or minor inflammation. Many recognize it under the name Domeboro, often used in soak or compress form for skin troubles like poison ivy, bug bites, and athlete’s foot. The FDA gave aluminum acetate an okay for over-the-counter use, a nod few ingredients get without years of scrutiny.
Looking deeper, I notice healthcare workers and pharmacists reach for this product when someone walks in with weepy, irritated skin. It works by drying oozing blisters and calming down itching. What sets it apart? This isn’t a harsh antiseptic. Instead, it’s a gentle salt solution made by mixing aluminum salts and acetic acid. Hundreds of urgent-care visits later, its track record for mild reactions stands strong.
Skin Safety—What the Science Says
Some worry about the mention of “aluminum”—the same metal that stirs debate around deodorants and Alzheimer’s disease. The levels in aluminum acetate, though, don’t sink into your system much at all. Research published by the Journal of Dermatological Treatment finds that topical use of aluminum acetate rarely causes harm if directions get followed. The occasional side effects? Mild dryness or stinging when applied to broken skin, especially for folks with eczema or allergies. I’ve seen a handful of cases where someone’s skin got a little red or flaky, but those incidents passed quickly once they switched products.
Parents sometimes use aluminum acetate on kids, especially in summer rash season. Pediatricians suggest it because of its gentle action, reminding everyone not to leave soaks on for too long or use on open wounds. The precise concentration—usually around 5%—is important. If mixed at home, using water that’s too hot, too strong, or contaminated can bring more risk than relief. Always stick with pharmacy-prepared solutions if there’s any doubt.
Where Things Can Go Wrong
Most problems with aluminum acetate don’t start with the formula—they start with how people use it. Over-soaking or covering treated skin tightly can trap moisture and worsen irritation. Some users try making their own version by combining random “DIY” internet recipes, which ends up making sensitive skin burn or peel. Another hiccup comes for anyone allergic to aluminum compounds, though this is rare. If your skin reacts to antiperspirants, play it safe and test a small spot first.
Long-term exposure causes little concern for most adults and children, especially compared to steroids or harsh antiseptics. Still, applying aluminum acetate to infected cuts or raw wounds can slow healing or sting fiercely. Always consult a doctor if you’re treating babies under six months, deep wounds, or a condition that isn’t clearly improving within a week.
Smarter Use and Alternatives
For itchy or oozing skin, aluminum acetate is a solid choice if you use it right: soak a clean cloth, wring it out, place it gently on the area, and limit sessions to 15-30 minutes. Don’t wrap with plastic or apply more than the label says—more isn’t always better. If signs of allergy or worsening rash show up, stop and talk with a professional. For folks sensitive to aluminum or who want to avoid it entirely, plain saline, colloidal oatmeal baths, or calamine can offer relief without the metal salts. As always, pay attention to ingredient lists and look for products backed by medical evaluation.
Aluminum acetate in a bottle won’t make headlines, but in my time in clinics and pharmacies, it holds up as a safe, effective tool—just respect the directions and be honest about what your skin can handle.
Looking at a Common Home Remedy
Walking into a pharmacy or scrolling through medical forums, aluminum acetate solutions pop up as an option when someone mentions ear infections. It’s sold as Burow’s solution or “Domeboro,” better known for soaking irritated skin. I remember long ago being told to use it for swimmer’s ear, and it’s still listed in some over-the-counter ear drops. People want fast relief from itchy, sore, or draining ears—so it’s important to ask: Does aluminum acetate work, and is it safe for this job?
How Aluminum Acetate Works
Aluminum acetate comes from mixing aluminum salts with acetic acid. In medicine, the solution cools and dries inflamed tissue. Dermatologists use it to help rashes and bug bites. For ear care, the drying action draws out moisture trapped in the canal, which stops bacteria and fungus from growing wild. Doctors have recommended aluminum acetate soaks for decades because dry skin doesn’t lead to infections the way soggy skin does.
Ear Infection Types and the Real Evidence
Painful, swollen, or draining ears often get labeled “ear infection,” but not all infections act the same. Outer ear infections (otitis externa or swimmer’s ear) are not rare, especially among swimmers and kids. Here, the canal gets too moist. Bacteria, like Pseudomonas and Staph, love wet skin. Fungal infections sometimes take hold, too. Inner and middle ear infections, the ones behind the eardrum, grow from viruses and bacteria, not from dampness in the canal.
Aluminum acetate ear drops have support from old and new clinical advice—mostly for “swimmer’s ear.” A 2021 review in the journal JAMA Otolaryngology listed aluminum acetate as effective for mild infections. Guidelines from groups like the American Academy of Otolaryngology say doctors can use it if pus hasn’t blocked off the canal or if the eardrum hasn’t burst. Its main job is as a “drying” and astringent drop, not as an antibiotic. I’ve seen people with mild, early irritation get quick relief this way.
No strong evidence exists that this solution does much for middle ear infections or anything behind an intact eardrum. Antibiotic or antifungal treatment will still be necessary for tougher bugs or serious cases. Doctors worry about the eardrum. If it’s ruptured, aluminum acetate in the middle ear can trigger hearing damage. I’ve seen some people use ear drops without knowing their drum has a hole. That will only make things worse.
Safety Issues and Drugstore Advice
Pharmacies still stock aluminum acetate ear drops and irrigating solutions. But the warnings should not go ignored. Most packages say not to use them with ear tubes, recent surgery, or signs of a ruptured drum (like flowing yellow or green pus, or sudden hearing loss). Allergic reactions are rare but possible, and alcohol-based versions can sting sensitive skin. The FDA classifies aluminum acetate as generally recognized as safe for short use. Still, long-term or repeated dousing can dry out the canal and make skin crack or itch more, setting up future infections.
Finding the Right Solution
A little knowledge goes far when it comes to at-home ear care. For mild swimmer’s ear, drying drops like aluminum acetate may help stop a simple problem before it grows out of hand. Stubborn, spreading, or visibly draining infections call for a real medical visit. Ear pain with fever, hearing loss, or drainage needs a professional to check if the eardrum sits intact.
People who swim, sweat, or wear hearing aids every day can keep ears healthy by tilting out water, using a towel, or using a hair dryer on low and cool—not cotton swabs or sharp objects. Doctors can give tailored guidance based on the story, the symptoms, and the full exam. Aluminum acetate has a place in ear care, but not for every case, and not forever.
Aluminum Acetate Isn’t a Cure-All
People lean on aluminum acetate for its soothing touch on skin irritations. I see it pop up in pharmacies for rashes, athlete’s foot, insect stings, and even poison ivy. Soaking a sore foot or dabbling a moist compress brings relief. Doctors, pharmacists, and people at home turn to it because it’s affordable and found just about anywhere. Even a mild, over-the-counter drug deserves some scrutiny—our skin absorbs what touches it, and the choice of treatments plays a role in overall wellbeing.
Typical Side Effects
Everyday use of aluminum acetate sticks to the same story: minor irritation at the site where it’s applied. Maybe it stings a bit, or you spot some redness. Most folks just shrug it off and keep going. The fact that it’s an astringent means it dries, tightens, and cools—the reason you end up feeling relief, but also why skin sometimes gets too dry or flaky. Overuse can make the problem worse instead of better.
On rare occasions, someone will break out in hives, notice more swelling, or feel itching beyond what prompted treatment. That’s when you should put the bottle down and call for help. The reaction could reveal an allergy, and no over-the-counter comfort is worth a trip to the ER for trouble breathing.
Not Just Skin-Deep
Aluminum, as a metal, usually gets a bad rap. There’s ongoing debate about its connection with Alzheimer’s disease. Scientific evidence on that link remains murky; using topical aluminum acetate hasn’t been shown to affect the body the same way as ingesting aluminum. The skin, for the most part, blocks absorption, but that’s not a guarantee. Broken or open skin may let more in, so lay off if there’s bleeding or fresh wounds unless a doctor says otherwise.
Risks for the Few
Anyone with kidney disease should think twice about aluminum-based remedies. Kidneys filter out metals, and if they lag behind, the body struggles to clear out extra aluminum. That situation can build up, causing bigger health concerns. It’s a good habit to flag any chronic health issues with your pharmacist or doctor before starting even simple treatments. Kids and teens with weak immune systems land in the same category—they need extra eyes on what goes onto their skin.
Reducing the Chance of Trouble
Doctors recommend using just enough to cover the rash or itchy patch, never slathering the entire body or tightly wrapping bandages over treated skin. Let the skin breathe; the aim is to calm irritation, not trap moisture and chemicals. If a rash spreads, or symptoms last more than seven days, that’s the cue to ask a professional. Reading the label, especially for those prone to allergies, steers people away from possible triggers.
Alternatives on the Shelf
Plenty of options compete for attention in drugstores—plain old calamine lotion, hydrocortisone, or even gentle oatmeal baths bring relief for most skin complaints. Selecting a home remedy based on personal health and skin type rather than brand loyalty makes more sense. If a product starts causing more problems than benefits, it’s time to try something else or check with a doctor.
| Names | |
| Preferred IUPAC name | aluminum triacetate |
| Other names |
Alumolate
Aluminum diacetate Aluminum ethanoate Acetic acid, aluminum salt |
| Pronunciation | /əˌluː.mɪ.nəm əˈsiː.teɪt/ |
| Identifiers | |
| CAS Number | [139-02-6] |
| Beilstein Reference | 1904327 |
| ChEBI | CHEBI:32436 |
| ChEMBL | CHEMBL1201571 |
| ChemSpider | 21569873 |
| DrugBank | DB09449 |
| ECHA InfoCard | 03e9dee5-69e1-4c43-96c6-40fbf4c7e438 |
| EC Number | 200-143-0 |
| Gmelin Reference | 1145 |
| KEGG | C14349 |
| MeSH | D000593 |
| PubChem CID | 8906 |
| RTECS number | AJ4300010 |
| UNII | 9Z8M85RDJ9 |
| UN number | UN1435 |
| CompTox Dashboard (EPA) | DTXSID4023574 |
| Properties | |
| Chemical formula | C6H9AlO6 |
| Molar mass | 204.113 g/mol |
| Appearance | White powder or crystals |
| Odor | Odorless |
| Density | 2.44 g/cm3 |
| Solubility in water | Soluble |
| log P | -2.39 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 23.1 |
| Basicity (pKb) | 3.5 |
| Magnetic susceptibility (χ) | −20.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.464 |
| Dipole moment | 3.8 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1441.5 kJ/mol |
| Pharmacology | |
| ATC code | S02AA02 |
| Hazards | |
| Main hazards | May cause skin, eye, and respiratory irritation. Harmful if swallowed. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P301+P312, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362 |
| Lethal dose or concentration | LD50 (oral, rat): 6207 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 6,200 mg/kg |
| NIOSH | SDC7800200 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg(Al)/m³ |
| Related compounds | |
| Related compounds |
Aluminum sulfate
Aluminum chloride Aluminum hydroxide Aluminum potassium sulfate Sodium acetate Potassium acetate |



