1-Ethyl-3-methylimidazolium Acetate: Commentary on a Modern Ionic Liquid
Historical Development
Just a few decades ago, 1-ethyl-3-methylimidazolium acetate—often called EMIM Acetate—remained a novelty in the world of chemistry. The imidazolium family only began to draw serious attention in the late twentieth century, as researchers searched for alternatives to volatile and hazardous organic solvents. From the experience of watching industrial labs becoming nervous over chemical emissions and waste, I noticed the shift towards greener solvents sparked serious investment. EMIM Acetate entered the scene as one of the first to offer broad solvency with a fraction of the environmental baggage. It became clear that ionic liquids like this one weren't just passing fads; their thermal and chemical stability opened doors in chemical engineering, pharmaceutical research, and materials science. In the late 1980s and early '90s, early adopters experimented with small scales, then moved quickly as the solvent's utility in biomass processing and catalysis turned heads. Balancing melting point, viscosity, and the ability to dissolve tough substrates changed a few careers and started lasting partnerships between academia and industry.
Product Overview
EMIM Acetate is no ordinary solvent. Its structure—benefiting from an imidazolium ring carrying both ethyl and methyl groups, paired with the acetate anion—stands out for providing low volatility, non-flammability, and strong hydrogen-bonding capacity. The transparent and near-odorless liquid feels different from traditional organic solvents: heavier on the tongue and sticky if spilled. Sourcing EMIM Acetate for a lab means committing to safer storage and getting used to different cleanup protocols, which speaks volumes in labs packed with undergraduate assistants. The substance's wide liquid range, covering subzero temperatures to comfortably above the boiling point of water, brings chemical engineers the sort of flexibility to run reactions that would shut down with other solvents. The story here tracks lab safety, performance, and sustainability—all high-priority buzzwords among chemical manufacturers trying to lower their regulatory burdens.
Physical & Chemical Properties
Physically, EMIM Acetate lands in a unique place. With a melting point hovering just below room temperature and a boiling point pushing above 100°C at reduced pressure, it’s useful nearly year-round. The density falls close to 1.1 g/cm³, significantly denser than water, making phase separations easy to monitor. Its viscosity can slow down stirring speed in the flask, which catches new researchers off guard. Chemically, EMIM Acetate doesn’t evaporate into the air easily and resists catching on fire, which feels like a breath of fresh air when handling liters in pilot reactors. The imidazolium cation roams easily, thanks to the acetate’s ability to accept and donate hydrogen bonds. This means cellulose, lignin, and polar organic molecules jump into solution, a big deal for those working with plant waste or stubborn pharmaceuticals.
Technical Specifications & Labeling
Commercial suppliers standardize EMIM Acetate production to meet both purity and moisture criteria. Many batches arrive boasting purities above 98%, with trace water below 0.2%, because even minor contaminants can disrupt catalysis or interfere with enzyme stability. Labels state the batch number, synthesis route, and any residual halide content, critical for interpreting experimental anomalies later. Transport containers remind handlers of the high density, low flammability, and the need to seal tightly after use; leaving a flask open invites water absorption, ruining sensitive applications. Warehouse managers value its stability but know that mixing with strong acids or bases can break down the material quickly.
Preparation Method
Synthesizing EMIM Acetate usually starts with simple ingredients—1-methylimidazole, ethyl chloride or ethyl bromide, and sodium acetate. The process consists of alkylating the imidazole core, then neutralizing with sodium acetate. Chemists choose their base and counterion carefully, as leftovers or by-products like sodium bromide or chloride leave residual inorganic salts. Recrystallization or distillation steps take patience, and deionized water washes drag out any hydrophilic impurities. Chemists often use vacuum drying or molecular sieves because EMIM Acetate loves to soak up water from the air, and water changes its physical properties.
Chemical Reactions & Modifications
This ionic liquid acts as both a solvent and a participant in chemical transformations. It can catalyze a wide range of organic reactions, particularly where strong hydrogen bonding or stabilization of charged intermediates is key. Occasionally, EMIM Acetate undergoes anion exchange, swapping acetate for another, like chloride or tetrafluoroborate, to adjust solubility or reactivity for a particular process. In large-scale cellulose dissolution or chemical pretreatment, the acetate anion sometimes reacts to form esters or acetic acid, which experimentalists track to prevent batch-to-batch losses. Shuffling the side groups on the imidazolium ring leads to cousins of EMIM Acetate with different viscosities or toxicities, giving researchers a large toolkit.
Synonyms & Product Names
In catalogs and scientific papers, 1-ethyl-3-methylimidazolium acetate comes with several aliases. EMIM Acetate, [EMIM][OAc], or C6H11N2O2 often pop up. Some vendors prefer the slightly wordier "ethyl(methyl)imidazolium acetate." Depending on the supplier, labels and certificates mention the CAS number 143314-17-4, which helps prevent mix-ups with similarly named ionic liquids. Chemists call it by many names, but the properties tie back to the same robust solvent.
Safety & Operational Standards
From personal experience, managing EMIM Acetate involves respect but not the same nerve-wracking attention as substances that ignite or explode easily. Wearing gloves and goggles is non-negotiable. A single splash on the skin stings or dries out the surface after long exposure, and the liquid clings stubbornly to glassware. Labs store it in tightly sealed bottles away from acids or bases. Despite its low volatility, chemical hygiene protocols call for handling it under a fume hood, especially during heating or mixing. Disposal procedures demand separation from halogenated waste, mainly because the acetate anion can generate acetic acid if heated with strong acids. Regular monitoring for contamination helps keep batches fit for sensitive syntheses. Testing for breakdown products—through NMR or chromatography—forms part of many standard operating procedures.
Application Area
EMIM Acetate owes much of its buzz to successes in dissolving cellulose and other stubborn biomaterials. Wood pulping, biofuel processing, and recycling of biomass feedstock benefit sharply. Its ability to break down complex natural polymers without severe chemical conditions helps companies move away from toxic acids or bases. Pharmaceutical chemists prize the ionic liquid for extracting specific alkaloids or crystalline drug candidates, achieved by tuning the balance of solubility and selective precipitation. In catalyst development and homogeneous reactions, its stability under heat and relative inertness raise yields for tough transformations. Electrochemists use EMIM Acetate as a component in supercapacitor electrolytes, appreciating its low flammability and broad electrochemical window. Academic circles push it into enzymatic biotransformations and non-aqueous enzyme stabilization, hoping for a greener approach to fine chemical synthesis.
Research & Development
The steady flow of patents and papers surrounding EMIM Acetate speaks to its versatility. University teams and private labs keep tuning the cation or anion to fit emerging needs—whether for tougher battery electrolytes, protein extraction, or processing of plastics that stubbornly resist conventional solvents. Recent experiments focus on cutting manufacturing costs and scaling up operations. Bioengineers keep tweaking process conditions to improve cellulose-to-sugar conversions, aiming to crack the code on affordable cellulosic ethanol. I have seen teams testing hybrid solvents—blends of EMIM Acetate and other ionic liquids—to find the sweet spot between high solubility, low toxicity, and regulatory approval. Green chemistry advocates keep asking how to reclaim and reuse this ionic liquid in industrial operations, hoping to create near-zero-waste processes.
Toxicity Research
Toxicity drives a large wedge in the application debate. While EMIM Acetate usually rates as less harmful than traditional solvents, early-stage data and studies in aquatic environments suggest that its cation-anion combination affects cell membranes and aquatic life under high concentrations. Chronic exposure studies remain limited, prompting calls for more research before broad deployment in open systems. Precautionary limits now shape discharge standards, especially near manufacturing or biorefinery sites. Worker protection measures stay conservative. Handling guidelines recommend full skin and eye protection, plus careful storage to avoid accidental mixing with incompatible chemicals. With ongoing studies using model organisms and in vitro methods, the risk landscape could shift, affecting how widely the solvent gets adopted.
Future Prospects
Looking ahead, EMIM Acetate faces strong prospects. The push toward renewable resources and green chemistry offers its best opportunities—especially when regulatory pressure, consumer expectations, and cost dynamics converge. Cellulosic ethanol, green plastics processing, and sustainable pharmaceutical manufacturing stand to benefit. Advances in recycling and purification will bring production costs down, but the biggest hurdles remain toxicity profiles and lengthy regulatory paths before widespread acceptance in food or pharmaceutical processing. Ongoing R&D focuses on tailoring chemical properties to hit performance targets, studying long-term effects on workers and ecosystems, and pushing for scalable reuse cycles in industry. As the world gaps between chemical performance and environmental responsibility, the story of EMIM Acetate promises plenty of new chapters.
A Tool for Dissolving Biomass
Science over the last decade has produced some remarkably useful chemicals, and 1-ethyl-3-methylimidazolium acetate stands out in that mix. Research labs started using this clear liquid salt for one big reason: it breaks down plant matter better than almost anything else on the market. Cellulose, the tough stuff in wood and plants, puts up a stubborn fight against many common solvents. Pour this ionic liquid onto dried switchgrass or sawdust—suddenly, pulp turns soft, almost syrupy. The process saves time, cuts down on the need for harsh chemicals, and helps scientists unlock what’s inside plant cells.
Changing the Way We Make Biofuels
Trying to make ethanol or other liquid fuels from plant scraps? The old way—cooking with lots of acid or caustic soda—brings headaches and pollution. In pilot plants, researchers add 1-ethyl-3-methylimidazolium acetate to shredded corn cobs or wheat straw. The salt loosens up the cellulose, letting enzymes chop it into sugars that can ferment into fuel. The gains are real: higher sugar yields without the sludge and wastewater from traditional methods. Getting biofuels off oil and onto plant waste could cut greenhouse gases, especially with growing pressure to ditch fossil fuels.
Cleaner Solvent for Tough Chemistry Problems
In my grad-school days, mixing chemicals meant stacks of flammable solvents, each with warning stickers. Ionic liquids like this acetate offer a safer route—non-volatile, nearly odorless, and slow to evaporate. Chemists swap in these salts for volatile organics to reduce risk and cut down on air pollution in the lab. You don’t get headaches from the fumes, and spills clean up with little trouble. No wonder companies making new polymers or specialty plastics tested these salts for industrial-scale chemistry, aiming for cleaner, less hazardous reactions.
Recycling Old Paper and Textiles
Paper mills and textile plants face piles of waste: old books, torn clothing, or low-quality fibers clogging up landfills. With the right setup, 1-ethyl-3-methylimidazolium acetate separates the cellulose and lignin within that waste, letting manufacturers recover pure pulp for fresh products. The method beats chlorine bleach on environmental impact, leaving behind fewer toxic byproducts. Some projects re-spun recycled cotton, adding value to something often tossed away. More engineering remains before recycling plants can handle big volumes, but early trial runs give hope.
Scaling Up While Staying Sustainable
One headache keeps popping up: the high cost and sometimes laborious recycling process for the ionic liquid itself. Making these salts uses energy, and the price tag gets steep if recovered solvents escape during processing. Engineers tinker with closed-loop systems, aiming to grab every drop for reuse. To see these green solvents reach more factories, costs need to drop, and recovery has to improve. Regulators also expect companies to prove the chemicals are safe long-term, especially before using them on the massive scale the bioeconomy demands.
Keeping One Eye on the Future
Someone learning about 1-ethyl-3-methylimidazolium acetate might think it’s just another lab reagent. The reality feels bigger. Right now, this salt helps remake old stuff—plants, paper, textiles—into new, useful materials. It gives labs a cleaner way to break things down or build better molecules. More companies testing these solvents will drive prices down and open up everyday products with less waste and pollution. Fingers crossed that smart engineering, wise regulation, and the push for cleaner industry keep this chemical’s promise growing.
What Makes This Chemical Relevant Right Now?
Chemical laboratories and industrial sites have seen a surge in the use of ionic liquids. 1-Ethyl-3-methylimidazolium acetate, a mouthful for sure, has carved out a space as a solvent thanks to its ability to tackle tough biomass and improve cellulose processing. People often ask if safety matches the hype. Getting real answers takes more than product brochures or wishful thinking about “green” chemistry.
Personal Lessons Working with Chemicals
I’ve spent time in research labs handling bench-scale trials with dozens of organic solvents, including 1-ethyl-3-methylimidazolium acetate. A trait that stands out is its minimal vapor pressure, so there’s little stink and barely any fumes. You might think this makes it safe–out of sight, out of mind–but life teaches us not to trust what we can’t see. Gloves and goggles become second nature, not because of regulations, but from those moments where a single drop ate through a lab-bench label or left a persistent sticky sheen. Some ionic liquids look benign until accidental contact causes a chemical burn or rash hours later.
Digging into Toxicology
Published animal studies show that this solvent isn’t outright deadly in small exposures, especially compared to legacy industrial chemicals. The trouble starts with chronic exposure. Rats and rabbits exposed through skin or ingestion have reported organ changes, metabolic stress, and reproductive impacts. Researchers point to ionic liquids’ knack for disrupting cell membranes. Over time, even small exposures can cause bioaccumulation, especially in aquatic systems.
No credible scientist will call this chemical non-toxic. Regulatory bodies like the European Chemicals Agency stress that limited information does not mean safe. Peer-reviewed studies call for extra caution, reminding us that “low volatility” just hides some risks, it doesn’t erase them.
Environmental Persistence
Ionic liquids break down slowly in natural environments. Their charged structure resists degradation by water or sunlight. Wastewater treatment plants can’t easily remove them, so traces slip into streams and groundwater, threatening aquatic life. Small-scale spills add up. Local studies in Europe and Asia document declining microbial diversity in wastewater effluent exposed to these solvents.
Workplace and Lab Safety Culture
Over the years, I’ve watched the best labs never get too comfortable with new materials. A good crew never skips hazard assessments, especially for chemicals that break the old solvent playbook. Even chemicals with low fire risk need handling with respect. Ventilation, skin protection, and chemical-resistant aprons become as important with ionic liquids as with acrylonitrile or pyridine. Best practices call for dedicated waste collection and isolation from water streams.
Steps Toward Safer Use
People ask what can be done about undisclosed or under-researched risks. Straight answers begin with powerful data sharing between labs and regulators. Every industrial site needs protocols targeting these “mild but persistent” hazards. Routine environmental monitoring picks up trends early. Worker training and incident reporting lead to better risk maps. Above all, slow adoption of new chemicals helps engineers adapt as health implications become clearer over time.
Why This All Matters
Believing in chemistry’s power to solve big problems pays off only if we keep tough questions at the center. The green label on new solvents means little unless health and environmental answers get equal spotlight. 1-Ethyl-3-methylimidazolium acetate offers some great advantages in the lab, but its risks grow with overconfidence and underreporting. Caution and common sense, backed by data, set the best foundation for safe progress.
Why Storage Rules Matter for This Ionic Liquid
Anyone working with chemicals knows well that storage isn’t a box-checking formality. I’ve seen labs scramble to contain spills or deal with ruined stock—all from missed details. For 1-ethyl-3-methylimidazolium acetate, safe handling supports both efficiency and lab safety. This isn’t your run-of-the-mill solvent. The liquid's ionic nature gives it powerful dissolving strength, but it also means the chemical can pull in moisture from the air. Leaving a bottle open or loosely sealed turns it into a magnet for water. That’s not only wasteful—it throws off every reaction and analysis, costing labs time and cash.
Controlling Moisture: Your First Line of Defense
This liquid wants to soak up water at every turn. The open bench top isn’t an option for any length of time. I always keep containers tightly capped, ideally with a PTFE-lined lid. Even one forgotten half-closed bottle leads to expensive surprises. For my own peace of mind, glass is the go-to. Plastic will let traces of air slip through over time, especially at the cap threads.
Storage in a desiccator helps keep humidity at bay, and silica gel packs work well for backup. Whenever I’ve tried skimping and used untreated benchtop air, the next titration showed the mistake—impure solvent, wasted batch. Any lab going through containers slowly should consider repackaging into smaller bottles to prevent moisture sucking in during repeated openings.
The Danger of Heat and Light
Temperature swings kick off chemical breakdown, even if the bottle looks untouched. Refrigerators extend shelf life, but ordinary fridges in shared spaces come with frequent opening—moisture rushes in. Dedicated chemical refrigerators solve that, staying cool and dry. I always avoid freezing. Ice crystals in ionic liquids create layers and mess with homogeneity.
Fluorescent lighting or sunlight jars the solution over time, especially at the bottle rim. My own hard-learned lesson came from a bottle left near a sunny window, with yellow tint and odd-smelling residue after a week. Shelves away from direct light solve the problem. Opaque storage bottles pair well for extra protection.
Avoiding Cross-Contamination
Even meticulous users slip up on labeling and handling. I write the opening date and initials with each container—no exceptions. Pipettes and spatulas see use with more than one reagent during a hectic day, so clean and dry them before touching this ionic liquid. I’ve watched colleagues blame batch failure on supplier errors, only to realize it was tainted by some stray salt from an unlabeled transfer tool.
Spills need fast cleanup, because the residue tracks across benches and glassware. Keep paper towels and gloves on standby, and never reuse cleanup rags.
Safety First: Protecting People and Workspaces
The acetate anion in this liquid smells sharp and turns skin sticky. Even accidental splashes can draw in contaminants. I use nitrile gloves, not latex—they resist liquid penetration better. Good ventilation helps, especially in cramped academic labs. Fume hoods don’t just block smells; they also limit personal exposure if splashing happens.
Material safety data sheets point out the general risks, but real safety comes from habit. Taking shortcuts eventually backfires. Everyone benefits from regular refreshers—short, honest discussions build trust and make safety procedures stick.
Storing 1-ethyl-3-methylimidazolium acetate correctly takes effort up front, but the payoff in cleaner reactions and safe coworkers adds up over years. These routines make every batch more reliable.
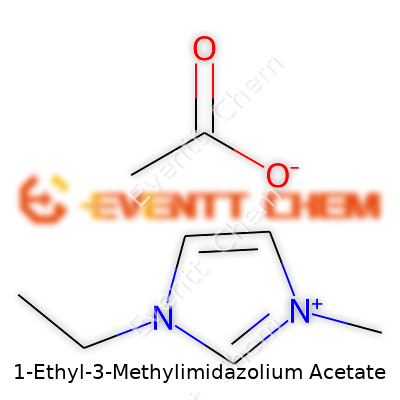
Unpacking the Chemical Formula
1-Ethyl-3-methylimidazolium acetate often goes by the shorthand [EMIM][OAc]. The formula shows up as C8H14N2O2. The molecule splits into two parts: the cation, 1-ethyl-3-methylimidazolium, and an anion, acetate. With the cation, chemists start with an imidazole ring—five atoms, three carbon and two nitrogen—then stick an ethyl group at the first nitrogen and a methyl at the third. For the acetate, think of a two-carbon chain with a carboxylic acid at the end, minus a proton.
Visualizing the structure helps. In 1-ethyl-3-methylimidazolium, the imidazole ring holds its core shape, almost like a hexagon with a gentle pinch. The ethyl group (–CH2CH3) hangs from one nitrogen, the methyl group (–CH3) dangles from the other. The acetate anion (CH3COO−) matches up close by, held by strong ionic interactions, but never actually forming a true covalent bond with the cation.
Why This Structure Makes a Difference
Students and researchers bump into EMIM acetate while looking for unusual solvents. The ionic character means this liquid barely evaporates, which straight away makes it safer for the lab bench, compared to volatile options like acetone or ether. Its structure lets it break down tough plant bonds, like cellulose, and makes it a bit friendlier for sustainable chemistry.
I’ve seen EMIM acetate in action in projects stripping lignin away from plant tissue or researchers attempting to recycle plastics. Its unique shape—bulky yet highly charged—lets it coax apart tangled biopolymers or plastics that can resist more common solvents. The lack of aromatic hydrocarbons or volatile groups cuts down on harmful fumes, giving those in the lab a welcome break from headaches and breathing masks.
Real-World Impact and Challenges
The world does not need any more hazardous waste, so less toxic, reusable solvents carry weight. EMIM acetate checked that box for a decade or more. The acetate anion, with its basicity, pries open hydrogen bonds in cellulose and other biopolymers, turning tough feedstock into something you might stir in water. Using ionic liquids for biomass treatment or recycling comes up again and again in environmental science.
Yet nothing’s perfect. EMIM acetate demands careful attention in large-scale processes. It can corrode steel or leach metal ions, triggering maintenance headaches in factories. Disposal needs real planning; though less harmful than methylene chloride, it won’t just break down in the local wastewater pond. Bringing these ionic liquids to market calls for balanced rules—making sure safety trumps speed.
Possible Solutions and Future Moves
Many chemistry departments train students to handle EMIM acetate for extraction, purification, or even simple synthesis. Supporting research with clear safety data sheets and strong ventilation goes a long way. For those working outside the lab, industry might consider developing recovery systems, pulling the ionic liquid out after each use, instead of turning it to waste. Green chemistry prizes such closed-loop practices.
As researchers dig deeper, new variations of ion pairs—maybe with even less toxicity or cheaper building blocks—could push past today’s options. For now, the clear formula and flexible structure of 1-ethyl-3-methylimidazolium acetate write it into the story of sustainable chemistry and resource recovery.
A Deep Look at Recycling in Modern Chemistry
1-Ethyl-3-methylimidazolium acetate—chemists know it as a popular ionic liquid. It’s earned a place in labs and factories because it dissolves cellulose, handles tough chemicals, and works in biomass processing. The real question is: can this expensive liquid get a second life after its first round of use?
Practical Realities of Reuse
Anyone working with this chemical quickly figures out it doesn't come cheap. You want to stretch every drop, not just for the budget but for the planet. After dissolving cellulose or helping separate plant material, the liquid doesn’t vanish into thin air. It holds on to water, bits of biomass, and whatever else it touched. Reusing it straight out of the reaction vessel won’t cut it.
Researchers and engineers have tried cleaning up used 1-ethyl-3-methylimidazolium acetate through filtration, evaporation, and distillation. Water content is a big hassle. This ionic liquid grabs water and won’t let go easily. Removing moisture demands effort and sometimes special vacuum drying, but it’s possible. Labs recover over 85% of ionic liquids through careful drying and purification. Some papers from the American Chemical Society highlight up to 90% recovery with good methods. This proves recycling isn’t just a theoretical idea. With some patience and setup, it works.
Quality Matters Over Time
Anyone who's reused solvents in a chemistry class knows—they don’t always come back the same way. Ionic liquids, including this one, can pick up tiny impurities—leftover sugars, or little slices of broken-down biomass. Over time, the liquid turns yellow or brown. Old, dirty solvent performs worse. Research in Green Chemistry shows that gradual buildup narrows the options for reusing the same ionic liquid. Simple filtration gets out solids, but dissolved impurities require deeper cleaning. Activated carbon filtration or treating with special resins can pull out some contaminants. Not all equipment is easy to set up at industrial scale. That’s one reason not every factory recycles all their ionic liquid.
Environmental and Economic Impact
The environmental story depends on how often the liquid gets recycled. Fresh batches take energy to manufacture, and most ionic liquids are tough to biodegrade. Every liter reused saves the energy and chemicals that go into fresh production. Waste management rules also matter here. Tossing contaminated solvent takes money and sometimes creates legal headaches. In Europe, the waste framework directive and other rules drive companies to recycle more when they can. The more times a batch of ionic liquid does its job, the less environmental footprint its life carries.
The Road Ahead
From my years in research labs, the drive for greener chemistry never fades. Nobody finds a perfect solution for every challenge yet, but steady improvements make a difference. Equipment gets better at pulling water out. Researchers tweak process steps to trap fewer impurities. Factories find ways to get five or ten cycles out of a single batch before it loses punch. There’s momentum, but also room to grow.
If future regulations offer more wiggle room for recycling solvents, or if global prices on ionic liquids rise further, expect more attention on developing next-level purification. Whenever a scientist or plant manager finds an affordable and easy way to reuse 1-ethyl-3-methylimidazolium acetate, they help shrink costs and cut down on waste. That’s good for business and for anyone hoping to build a more sustainable chemical future.
| Names | |
| Preferred IUPAC name | 1-ethyl-3-methyl-1H-imidazol-3-ium acetate |
| Other names |
EMIM Acetate
1-Ethyl-3-methylimidazolium ethanoate EMIM OAc |
| Pronunciation | /ˈwʌn ˈiːθɪl θriː ˈmɛθəl ɪˌmɪdəˈzoʊliəm əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 143314-17-4 |
| Beilstein Reference | 1431555 |
| ChEBI | CHEBI:135077 |
| ChEMBL | CHEMBL297442 |
| ChemSpider | 86979 |
| DrugBank | DB11126 |
| ECHA InfoCard | ECHA InfoCard: 100.237.378 |
| EC Number | 607-587-4 |
| Gmelin Reference | 78450 |
| KEGG | C22187359 |
| MeSH | D000070281 |
| PubChem CID | 2734164 |
| RTECS number | KI2000000 |
| UNII | 1O6UO28F1H |
| UN number | UN3485 |
| Properties | |
| Chemical formula | C8H14N2O2 |
| Molar mass | 170.21 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.11 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | -2.46 |
| Vapor pressure | 0.0000297 mmHg at 25 °C |
| Acidity (pKa) | 12.5 |
| Basicity (pKb) | pKb = 9.12 |
| Magnetic susceptibility (χ) | -62.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.500 |
| Viscosity | 57 cP (25 °C) |
| Dipole moment | 4.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 259.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -120.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3040.6 kJ/mol |
| Pharmacology | |
| ATC code | NO ATC CODE |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 102 °C |
| Lethal dose or concentration | LD50 Oral Rat 406 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1550 mg/kg |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-Ethyl-3-methylimidazolium Acetate is not established. |
| REL (Recommended) | 35 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Butyl-3-methylimidazolium Acetate
1-Ethyl-3-methylimidazolium Chloride 1-Ethyl-3-methylimidazolium Bromide 1-Ethyl-3-methylimidazolium Tetrafluoroborate 1-Ethyl-3-methylimidazolium Dicyanamide |



